Yesterday we saw two very significant data releases from Neurocrine Biosciences (NBIX) and ChemoCentryx (CCXI). Both releases were big disappointments, and caused significant devaluations of their respective companies.
This article will review both the NBI-98854 data and the CCX140 data, as well as the implications.
Neurocrine – Phase IIb Results of VMAT2 Inhibitor NBI-98854
NBI-98854 is a treatment for a neurological disorder known as tardive dyskinesia (TD). This orphan disease causes patients to produce repetitive, involuntary muscle movements. The causation of the disease is related to continuous use of antipsychotic drugs, although specific details on the mechanism of TD are unknown. The severity of the disease is measured using the Abnormal Involuntary Movement Scale (AIMS). Improvement is measured inversely with the score itself, so continuous reduction of the number implies that patients are improving.
The KINECT study, which studied 120 patients, failed to beat placebo at the 50 mg dosing at 2 and 6 weeks, although statistically significant improvements were seen at the 100 mg dose at both 2 and 6 weeks: 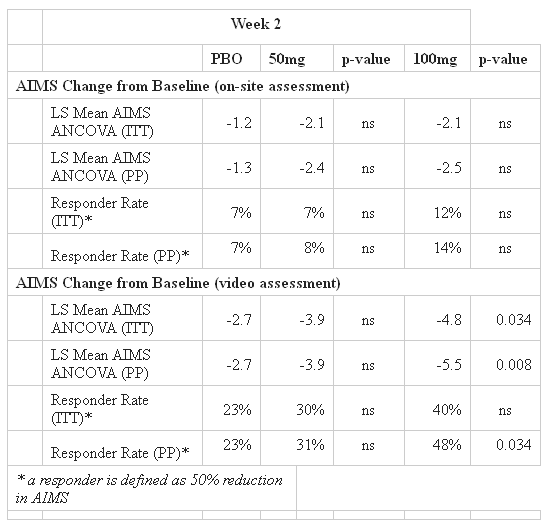
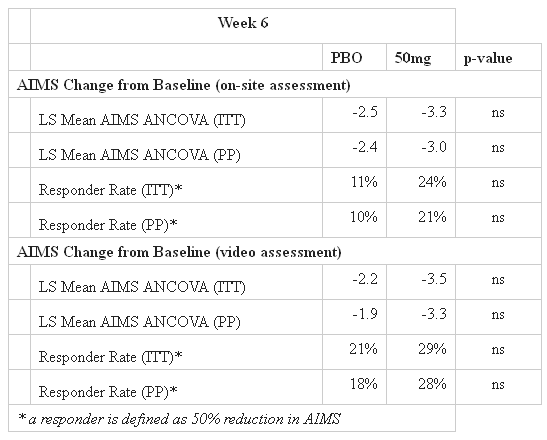
This led analysts to defend the stock against the drop, although many investors were concerned about the effects the results could have on the later clinical development of NBI-98854. This will also force the company to perform another Phase II trial at the 100 mg dose to confirm what was seen in KINECT.
Also, as stated by the company, the 100 mg dose was expected to be the maximum tolerated dose (MTD). Although the 100 mg patients appeared to tolerate the drug in this trial, previous data suggest that there may be some tolerability problems when a larger 100 mg trial is performed. This could lead to bigger problems with NBI-98854’s safety profile when the drug is eventually put up for final review.
The valuation of Neurocrine is still being supported by other pipeline assets like Elagolix, although it’s clear that this clinical trial was at least a partial failure. Although the clinical development of NBI-98854 can still be salvaged, its valuation deserved a reduction after results came in from KINECT. Since the bulk of Neurocrine’s valuation seems to be based around Elagolix and the partnership with Abbott Labs (ABT), we can infer that the majority of NBI-98854’s valuation was shaved off with yesterday’s move. It doesn’t seem that the market will give much of this back to NBIX unless future Phase II data at the 100 mg dose convinces investors that the drug can replicate its results from KINECT.
ChemoCentryx – Top-Line, Interim Phase II Results for CCR2 Inhibitor CCX140
CCX140 is an inhibitor of a chemokine receptor known as CCR2, which is being studied as a treatment for kidney disease caused by diabetes.
The company only released 12-week data from the 52-week Phase II study for diabetic nephropathy, although efficacy of the drug in the 5 mg CCX140 arm is falling below expectations. At week 12, the company was hoping to see improvements – as measured by reductions in proteinuria – of at least 20% relative to placebo (standard of care).
Although the data were incomplete, investors were not happy to see another clinical trial failure in the cards. Note that ChemoCentryx failed a Phase III study last month (SHIELD-I) with flagship drug Vercirnon, although partner GlaxoSmithKline (GSK) will take the drug through three more late-stage trials.
Much of the hype around ChemoCentryx is centered around the chemokine inhibition platform, which explains why top-line interim results could have such a profound impact on the valuation of CCXI. The data, while disappointing thus far, do not conclusively determine whether or not CCX140 underperforms current diabetic nephropathy treatments. Still, this uncertainty continues to unnerve large numbers of CCXI shareholders.
Investors who are interested in ChemoCentryx should be looking for full Phase II trial data for CCX140, which will be available in the second half of 2014. The results of this trial will more clearly determine whether chemokine inhibition is valuable to the pharmaceutical industry, and should have an enormous impact on shares of CCXI when they arrive.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Neurocrine, Chemo Centryx: Data Disappoints, Cause Devaluations
Published 09/12/2013, 01:36 AM
Updated 07/09/2023, 06:31 AM
Neurocrine, Chemo Centryx: Data Disappoints, Cause Devaluations
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
