As a general rule, the most successful man in life is the man who has the best information
When most of us suffer a cut cells in the blood, called platelets, go to where the cut is, plug the hole and stop the bleeding. While the platelets are plugging the hole they release chemicals that attract more of the ‘sticky’ platelets and twelve (numbered using Roman numerals I through XII) proteins in the blood known as clotting factors are activated. These proteins mix with the platelets to form fibers which make the clot stronger and stop the bleeding.
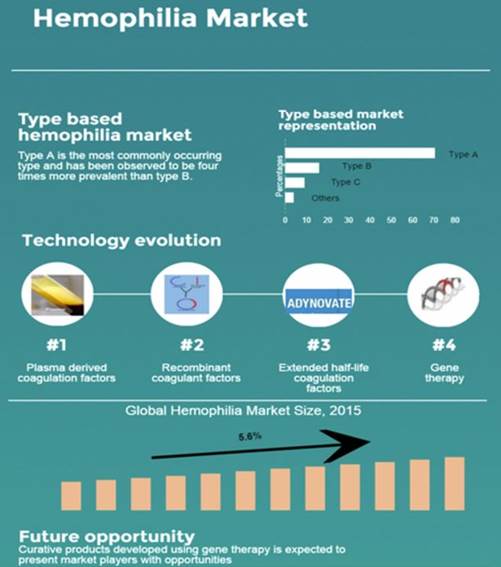
Having too little of factors VIII (8) or IX (9) is what causes hemophilia. A person with hemophilia will lack only one factor, either factor VIII or factor IX, but not both. There are two major kinds of hemophilia: hemophilia A, which is a factor VIII deficiency; and hemophilia B, which is a factor IX deficiency.
Hemophilia is a genetic disorder which means it's the result of a change in genes that was either inherited (passed on from parent to child) or occurred during development in the womb. Although it is mostly passed down from parents to children, about 1/3 of cases are caused by a spontaneous mutation, a change in a gene. All races and ethnic groups are equally affected by hemophilia A. The disease almost always affects males but can also affect females.
Many people believe that hemophiliacs bleed a lot from minor cuts but external wounds are usually not that serious. Much more serious is internal hemorrhaging that can take place in joints (especially knees, ankles and elbows) and into tissues and muscles. Bleeding can also occur in vital organs putting a hemophiliac's life in danger.
Although effective treatment of the symptoms is available, there is no cure for hemophilia A at present and therapy has to be individualized to specific patients. Patients have to get lifelong infusions with recombinant factor VIII (rFVIII) several times a week to compensate for the missing clotting factor.
The global total hemophilia market was valued at US$ 9.3 billion in 2015. Approximately 20,000 people in the United States, 10,000 in Europe and approximately 2,500 in Canada have a moderate or severe form of hemophilia A. Annual costs for the treatment of the disease for each patient may range from US$60,000 to US$260,000 for a total cost of between $2-5B per year just in North America and Europe.
Grand View Research
The Horizon 2020 program
Horizon 2020 is the largest European Union (EU) research and innovation program ever undertaken with nearly €80 billion of funding available over the seven years between 2014 to 2020. Horizon 2020 promises breakthroughs, discoveries and world firsts by taking great ideas from the lab to the market, for example in the field of personalized medicine providing novel therapies such as gene or cell therapy.
HemAcure project, a novel personalized medicine curative therapy
An international research consortium, under the name HemAcure unites scientific academic institutions from Germany, Italy, the UK and Sernova Corp from Canada.
The following institutions are involved in HemAcure:
- ARTTIC, a Munich-based enterprise that specializes in the management of EU-funded collaborative research projects, is in charge of project management.
- The Department of Tissue Engineering and Regenerative Medicine of the Wuerzburg University Hospital is responsible for isolating the cells.
- The Università del Piemonte Orientale (Italy) is developing, optimizing and performing the gene correction of the patient cells for expression of the Factor VIII therapy.
- Scientists from Loughborough University (UK) are focussing on the manufacturing process and safety testing.
- Sernova (V:SVA) a Canadian public company, is responsible for conducting the preclinical safety and efficacy studies with the Factor VIII producing cells in its proprietary Cell Pouch™ using a model of hemophilia developed by consortium partner Universita del Piemonte Orientale (Italy) in preparation for clinical trials.
- The quality management (GMP processes) is being monitored by IMS - Integrierte Management Systeme in Heppenheim, Germany. The company acts as a point of contact for international projects in the pharmaceutical and medical engineering sector.
The overall objective of the HemAcure project is to develop and refine the tools and technologies for a novel, curative ex vivo (outside the body) prepared cell based therapy to treat hemophilia A that should ultimately lead to improved quality of life for patients. The EU’s Horizon 2020 programme has stage funded the HemAcure project with €5.5 million (Cdn$8.06M, US$6.3). The most recent tranche of funding has just been approved based on the encouraging results to date.
The consortium’s idea: A personalized medicine solution using the patients' own cells (remember each patient has to have individualized therapy) which are genetically modified outside the body to produce the missing clotting factor using precursor cells of endothelial cells flowing in the bloodstream. After modification these cells are transplanted back into the patient's body in Sernova Corp’s Cell Pouch™.
After Sernova’s Cell Pouch™ is implanted in the body and forms its unique vascularized tissue chambers, the genetically modified cells are then transplanted into the vascularized chambers and are expected to continuously produce the clotting factor and release it into the bloodstream for a long period of time. This should mitigate the disease's impact noticeably, increase the patients' quality of life and reduce the overall cost of therapy.
Sernova Corp. (TSX-V: SVA) (OTCQB: SEOVF) (FSE: PSH), is a Canadian publically traded, clinical stage, regenerative medicine company developing an implantable, scalable device, the Cell Pouch System™ and therapeutic cells for the treatment of diseases such as diabetes, and hemophilia.
Sernova’s Cell Pouch™ forms a natural vascularized environment for long-term survival and function of the therapeutic cells which release into the bloodstream required but missing proteins or hormones.
Sernova’s Cell Pouch™ technology would be beneficial if it provided a simple reduction in the number of therapeutic injections a patient must take; however, there is the possibility that it could even essentially ‘cure’ the disease through natural release and regulation of the therapeutic proteins or hormones.
“Sernova has developed its proprietary highly innovative Cell Pouch technologies for the placement and long-term survival and function of immune protected therapeutic cells. It has proven to be safe and efficacious in multiple small and large animal preclinical models and has demonstrated safety alone and with therapeutic cells in a clinical trial in humans for another therapeutic indication (diabetes – editor). We believe the Cell Pouch platform is the first such patented technology proven to become incorporated with blood vessel enriched tissue-forming tissue chambers without fibrosis for the placement and long-term survival and function of immune protected therapeutic cells.” Sernova News Release, Marketwire – July 24, 2017
Sernova is today a relatively unknown pure regenerative medicine play that has partnered their Cell Pouch™ with a network of academic cell therapy research and development partners. Below is a HemAcure consortium approved news release issued by Sernova Corp. on Monday July 24, 2017.
It’s your authors opinion ‘relatively unknown’ is a term that will shortly no longer apply to Sernova Corp.
Sernova-HemAcure Consortium Announce Significant Progress in Development of ‘First in World’ Regenerative Medicine Therapy for Treatment of Hemophilia A Patients
Breakthrough scientific progress is made in development of a disruptive personalized regenerative medicine therapy within Sernova’s Cell Pouch(TM) for treatment of Hemophilia A validated by European Commission’s confirmation of next stage of funding of the €5.6Million EU Horizon 2020 Grant Award to the HemAcure Consortium
LONDON, ONTARIO – (Marketwire – July 24, 2017) – Sernova Corp. (TSX-V: SVA) (OTCQB: SEOVF) (FSE: PSH), a clinical stage regenerative medicine company, announced today significant scientific progress achieved in the development of a ‘first in world’ personalized regenerative medicine therapy for the treatment of Hemophilia A patients by the HemAcure Consortium and confirmation of the second phase of funding of the Consortium by the European Commission.
The therapy being developed by international scientific Consortium members consisting of three European academic institutions, an enterprise for quality management and Sernova Corp is to treat severe Hemophilia A, a serious genetic bleeding disorder caused by missing or defective clotting factor VIII in the blood stream. This therapy consists of Sernova’s implanted Cell Pouch(TM) device transplanted with therapeutic cells, corrected to produce Factor VIII at a level sufficient to significantly reduce the side effects of the disease and improve patient quality of life.
“The international HemAcure Consortium team members are pleased with the ground breaking scientific advances achieved at this point and are on track for this regenerative medicine solution to advance into human clinical evaluation,” remarked Dr. Philip Toleikis, Sernova President and CEO.
Toleikis added, “Sernova’s Cell Pouch platform technologies are achieving important world first milestones in both diabetes and now hemophilia, two significant clinical indications which are being disrupted by its regenerative medicine approach aimed at significantly improving patient quality of life.”
“We are thrilled with the approval by the European Union of the next stage of funding for the HemAcure program based on our quality interim report. This is a strong validation of the Consortium’s dedication and teamwork and the importance of this regenerative medicine approach,” said Dr. Joris Braspenning, HemAcure Program Coordinator.
In summary, the following ground-breaking developments have been achieved by the Consortium:
- A reliable procedure has been implemented to isolate and maintain required endothelial cells from a sample of the patient’s blood.
- Using a novel gene correction process, the cells have been corrected and tuned to reliably produce the required Factor VIII to treat Hemophilia A.
- The cells have been successfully scaled up to achieve the required therapeutic number, and cryopreserved for shipping and future transplant into the implanted Cell Pouch.
- A preliminary study confirmed survival of the Factor VIII corrected human cells injected into the hemophilia model, achieving sustained therapeutic Factor VIII levels. This preliminary work is being used to aid in dosing of these cells in the Cell Pouch.
- Safe Cell Pouch surgical implant and cell transplant procedures have been developed in the hemophilia A model in preparation for use in hemophilia patients.
- Development of Cell Pouch vascularized tissue chambers suitable for Factor VIII producing cell transplant has been demonstrated in the hemophilia A model, expected to mimic the predicted findings in human patients.
- In combination, this work is in preparation for safety and efficacy studies of the human hemophilia corrected Factor VIII producing cells in the Cell Pouch in a preclinical model of hemophilia.
This combination of advances by the HemAcure team represents a ‘first in world’ achievement towards developing a regenerative medicine therapy for the treatment of severe hemophilia A patients.
“In this regard, these fundamental advancements have set the stage for further optimization and implementation of cell production processes under controlled GMP conditions,” stated Martin Zierau, IMS member consortium team leader responsible for coordination of GMP processes.
With Factor VIII corrected cells, studies are ongoing to optimize cell dosing within the Cell Pouch and for study of safety and efficacy of hemophilia corrected Factor VIII cells in the hemophilia model. These studies are in support of the current extensive regulatory package already assembled for the Cell Pouch in anticipation of human clinical evaluation of the Cell Pouch with hemophilia corrected Factor VIII producing cells.
A Big Deal
Any discussions regarding advancing HemAcure’s plan, and more funding from Horizon 2020, had to be centered around success in these three areas:
- CELLS ARE PRODUCING FACTOR VIII: The Consortium has successfully developed the process for isolating and maintaining the required cells from a sample of patient’s blood. Using a special technique these cells have been corrected and tuned to produce Factor VIII on a constant basis.
- CORRECTED CELLS HAVE BEEN SCALED UP: The corrected cells have then been multiplied to demonstrate that the required number of cells can be produced. After testing, batches of corrected cells have been frozen, stored for later transplantation and successfully shipped, thawed and recovered. With further optimization and GMP production, this being the process anticipated to be used for future treatment of patients with hemophilia A.
- CELLS PRODUCING THERAPEUTIC BLOOD LEVELS OF FACTOR VIII: In further preclinical tests, in a preliminary study, Factor VIII producing cells have been shown to produce therapeutic blood levels of Factor VIII. Studies have already shown that the Cell Pouch can produce vascularized tissue chambers in the hemophilia model and further studies will optimize dosing of hemophilic patient corrected cells that will then be transplanted into the Cell Pouch™ for evaluation of safety and efficacy in the preclinical model of hemophilia.
Conclusion
Being that all the companies in the HemAcure consortium are private, except SVA, and that they plan on ‘bringing breakthroughs, discoveries and world firsts from the lab to the market’ might not Sernova be a great way to leverage this in your portfolio?
And SVA is no one trick pony, the company is a leader in the regenerative space with their Cell Pouch™ and upon FDA clearance plan to initiate clinical trials in the United States for diabetes – expected to start patient enrollment this fall.
Add in developing local immune protection technology within the Cell Pouch™ and the company’s very own glucose responsive stem cell technology, you can see why your author thinks Sernova Corp might just be the best regenerative medicine pure play out there.
All of these reasons are why Sernova Corp. is on my radar screen. Is SVA on yours?
If not, maybe it should be.
