Results of a ‘real-world’ study and an independent economic analysis confirm that Sativex is an efficacious and cost-effective therapy for MS spasticity. We see potential for GW Pharma’s (GWP.L) licensing partners (Almirall, Bayer, Novartis) to use these data in their ongoing pricing/reimbursement discussions and marketing efforts. Moreover, the study findings provide greater confidence in our peak sales forecast for Sativex in MS spasticity of £64m.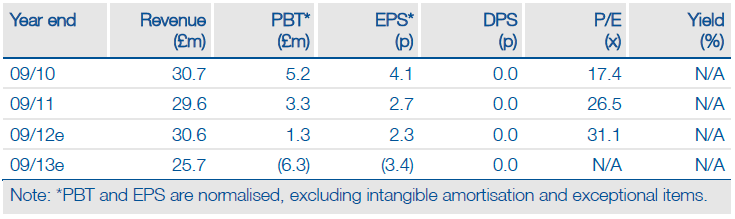
Sativex is effective in real-life setting…
Positive findings from a German real-world study of Sativex for multiple sclerosis spasticity (MSS) have been presented at leading MS conference, ECTRIMS. Results of the 300-patient, observational MOVE 2 trial showed that use of Sativex under reallife conditions can effectively relieve moderate-to-severe MSS (the drug’s approved indication). Sativex provided initial relief in 75% of previously resistant patients, with 41% reporting a clinically relevant (≥30%) reduction in spasticity after three months, mirroring the results of pivotal registration studies. As such, we see potential for GW Pharma’s licensing partners (Almirall, Bayer, Novartis) to use the MOVE 2 data to support ongoing pricing/reimbursement discussions and marketing efforts.
…and is also cost effective
Complementing this real-world data, an independent pharmacoeconomic analysis has concluded that Sativex is cost effective for MSS in Germany and Spain (Slof et al, 2012). The costs and quality-adjusted life years (QALY, a measure of quality and quantity of life) associated with current spasticity management were compared to Sativex. Results showed Sativex was cost effective over a five-year period in both countries, with low incremental costs and QALY gains compared to standard treatments. This was seen because reduced spasticity in MS patients led to lower consumption of healthcare resources (physiotherapy, medications).
Valuation: DCF-based valuation of £200m
While the real-world data and cost-effectiveness analysis have no immediate impact on our valuation or forecasts, they provide further support for our peak sales forecast for Sativex in MS spasticity of £64m. Greater than expected market share/growth and new indications for Sativex, clinical progress in cancer pain or developments with Otsuka (a second US Sativex indication or licensing deal on CNS/cancer candidates from the research collaboration) represent upside to our base-case valuation.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
GW Pharmaceuticals: Sativex's Efficacy Could Move the Dial
Published 10/18/2012, 03:47 AM
Updated 07/09/2023, 06:31 AM
GW Pharmaceuticals: Sativex's Efficacy Could Move the Dial
MOVE-ing the dial
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.