Many biotech traders and investors are excited, and maybe a bit anxious about the upcoming PDUFA action date for Exelixis’ (EXEL) cabozantinib in the treatment of Medullary Thyroid Cancer (MTC), which is scheduled for November 29th. Due to an unexplained cancellation of the actual discussion and vote on cabozantinib’s NDA for the treatment of MTC at the FDA’s Oncological Drugs Advisory Committee meeting (originally scheduled to take place between November 8th and 9th), the market will actually be heading into a FDA decision with nothing more than the related phase III clinical trial data that Exelixis released earlier in the year, along with other studies that supplement the drug’s efficacy and safety profile in cancer treatment.
Cabozantinib did meet its primary endpoint in the EXAM trial, and demonstrated statistically significant improvements in the PFS (progression-free survival) of cabozantinib treated MTC patients versus placebo. Due to its orphan drug designation for MTC, and the priority review status for the NDA granted on July 30, 2012, it seems unlikely that we’ll see a non-acceptance of cabo’s NDA in its current form. While it’s true that the FDA likes to be cautious, orphan drugs tend to be treated well.
Still, there is clearly some disagreement amongst traders about EXEL at this point, especially since short positions in EXEL have actually been on the rise in the last few months. Since the end of August 2012, the total number of shares short managed to jump from 35 million to the latest estimate of 40.8 million despite steep declines in trading volume in the same period. At this point, bears are confidnetly holding 25% of open shares for themselves.
In spite of the increased short interest, bulls have brought the stock price of EXEL above the psychologically important $5/share mark. While this doesn’t have any real effect on Exelixis as a company, there is a strange bias against stocks below $5/share, due to their classification as “penny stocks.” Due to this quirky effect, EXEL would probably gain some support from the bull side if it were to stay north of $5/share for a prolonged period of time. To demonstrate the importance of the $5/share mark, especially for EXEL, I included the chart below: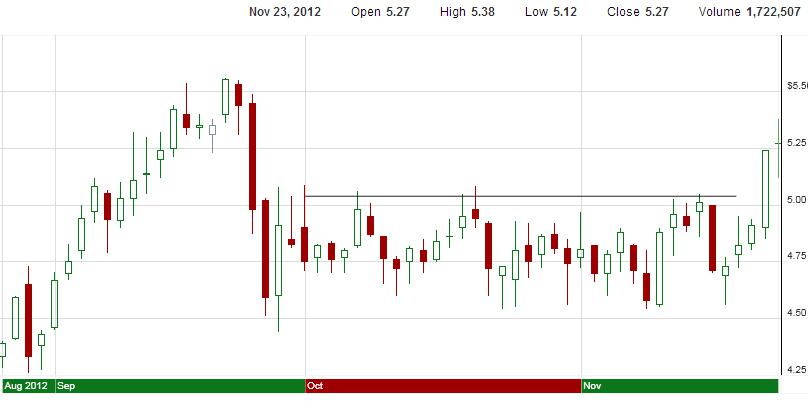
EXEL made a prominent break of what was formerly considered a “price ceiling,” and is now poised to recover a lot of lost ground from September. Given the large short interest on the stock, traders that are fond of rallies that are fueled by short-covering could consider the stock as a potential play, although the potential for an FDA rejection of cabo’s NDA for the treatment of MTC does introduce some risk into the equation.
Exelixis also has interesting prices in its options market, which is seeing a fair amount of activity this month. Options offer a lot of control to shareholders, and can provide substantial risk-adjusted returns for every type of traders. Cautious shareholders of EXEL, for instances, could hedge their bullish bets on the stock by selling December call options at decently large premiums. Particularly attractive are the $7.00 calls, which would provide an extra $.15/share of income. In the unlikely event that EXEL breaches $7/share in December, the shareholder would still be rewarded with very substantial gains on the stock.
As I’ve stated in previous coverage of Exelixis, there is still more importance placed by the market on the potential sales revenue of cabozantinib in the treatment for metastatic castration-resistant prostate cancer (or mCRPC) due to the sheer size of that market, but the extremely long wait for COMET trial results will be quite painful for many. These phase III results will tell us more about cabozantinib in the treatment of mCRPC when the results are released in mid 2014 or so, which will pave the way for the drug’s NDA.
Unfortunately, with the results scheduled for 2014, EXEL traders will first have to react to the catalysts that are closer. The next big trade is coming on November 29th, and it will be a reaction to the FDA decision for cabozantinib’s very first NDA submission.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Exelixis Traders Brace For Upcoming FDA Decision
Published 11/27/2012, 12:22 AM
Updated 07/09/2023, 06:31 AM
Exelixis Traders Brace For Upcoming FDA Decision
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
