The FDA has reported three deaths post Edwards Lifesciences Corporation’s (NYSE:EW) decision to recall its cardiac device. The agency stated that the voluntary recall of the IntraClude intra-aortic occlusion device in May has now been categorized as Class I recall.
Shares of the company dipped 0.34% to $184.64 on Jul 2, 2019 following the news.
For investors’ note, Class I recall is the most stringent form issued by the regulatory authority, where use of defective devices may lead to grave injury or death.
Per an official release, FDA has received as many as 22 complaints related to balloon rupture or puncture. A Reuters report claims, “The FDA said that recall affects more than 750 devices in the United States.”
The Device at a Glance
The IntraClude intra-aortic occlusion device is used in patients undergoing cardiopulmonary bypass, where the device takes over the functions of the heart and lungs for a temporary period during surgery.
Per the FDA, the recall was made due to a risk of balloon rupture during use of the devices,which is likely to make the procedure lengthy and put patient’s safety at risk.
The IntraClude balloon burst may lead to severe health consequences. The incident may increase the patient’s duration on cardiopulmonary bypass as well as cause neurological damage, embolism, stroke and death.
Regulatory Issues Dent Prospects
In April, Edwards Lifesciences had to recall two of its Atrioseptostomy catheters. FDA marked these as Class I recalls based on the possibility of difficulty in balloon deflation after deployment, which may lead to fragmentation or detachment on attempted retrieval.
In this regard, Boston Scientific (NYSE:BSX) and Edwards Lifesciences have reached an agreement to settle all outstanding patent disputes between the companies in all global venues in January 2019. All pending cases or appeals in courts and patent offices between the two companies will be dismissed. The parties will not file patent disputes related to current portfolios of transcatheter aortic valves, certain mitral valve repair devices and left atrial appendage closure devices.
Needless to say, these regulatory hurdles and complex legal proceedings are exerting pressure on Edwards Lifesciences’ margins.
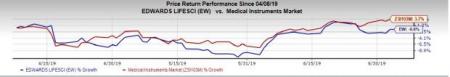
Price Performance
In the past three months, the stock has inched down 0.6%, against the industry’s rise of 3.7%.
Zacks Rank and Other Key Picks
Edwards Lifesciences currently carries a Zacks Rank # 3 (Hold).
Two of the better-ranked stocks in the broader medical space are Teleflex Inc (NYSE:TFX) and Penumbra (NYSE:PEN) . Each of these stocks currently carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Teleflex’s long-term earnings growth rate is expected to be 13.7%.
Penumbra’s long-term earnings growth rate is projected at 21.5%.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases. Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98%, +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Teleflex Incorporated (TFX): Free Stock Analysis Report
Penumbra, Inc. (PEN): Free Stock Analysis Report
Boston Scientific Corporation (BSX): Free Stock Analysis Report
Edwards Lifesciences Corporation (EW): Free Stock Analysis Report
Original post
Zacks Investment Research
