Deinove (PA:ALDEI) released its H119 results on 26 September 2019. The company reported progress with most of its projects in the pipeline, which is well diversified across a number of different programmes and technologies. We believe the antibiotic asset DNV3837 is the most valuable, as it is a pure drug development programme. Deinove has been preparing for the Phase II efficacy trial in C. diff infections and the first patient should be recruited in the coming weeks. The company also reported multiple developments in its bioactives portfolio. The first two commercialised products (Phyt-N-Resist and Hebelys) are expected to start generating sales this year. We have slightly increased our valuation of Deinove to €72m or €4.2/share.
Phase II trial with DNV3837 for C. diff to start
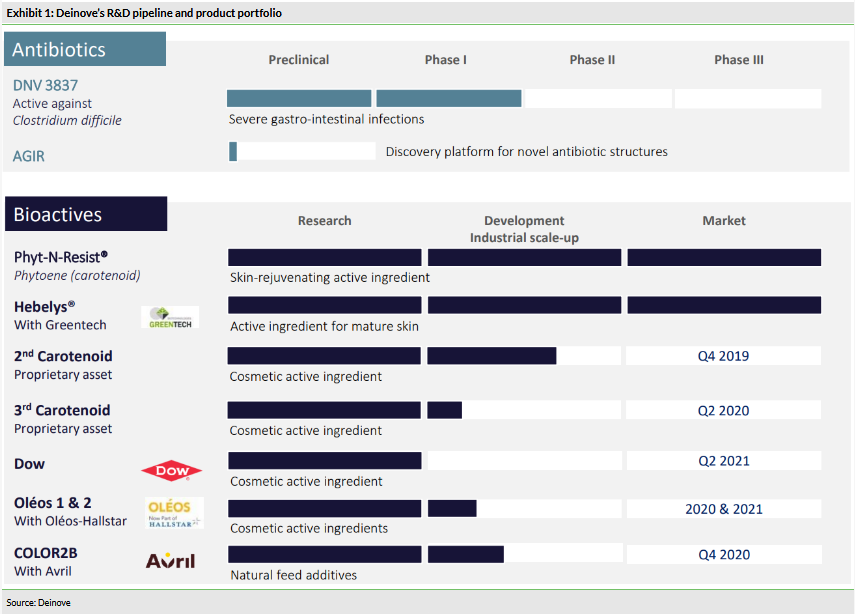
Deinove has continued preparations for the Phase II trial (NCT03988855) with its most advanced antibiotic candidate, DNV3837, a novel quinolonyl-oxazolidinone class molecule, for C. diff infection. The trial is being conducted in the US and the investigation centres have been selected. The first patient is expected to be enrolled in the coming weeks. The study should last for about 10–12 months. If positive, the next step would likely be a registration Phase III trial. DNV3837 has obtained a QIDP designation and Fast Track status from the FDA. The lack of a regulatory approved intravenous treatment option and growing incidence of C. diff present a significant opportunity for DNV3837, in our view.
Differentiated positioning of key bioactives
Phyt-N-Resist (100% pure phytoene) and Hebelys (Sphingomonas hydrophobicum) are the two cosmetics ingredients in Deinove’s bioactives portfolio and have been commercialised since early 2018. In addition to marketing activities, Deinove and its partners have been conducting preclinical and clinical studies to accumulate evidence and differentiate marketing claims. These efforts have resulted in greater understanding of the mechanisms of action of these products. So far, no sales have been booked, but Deinove has indicated that multiple potential customers have been evaluating samples of both ingredients and the first sales could start by the end of this year.
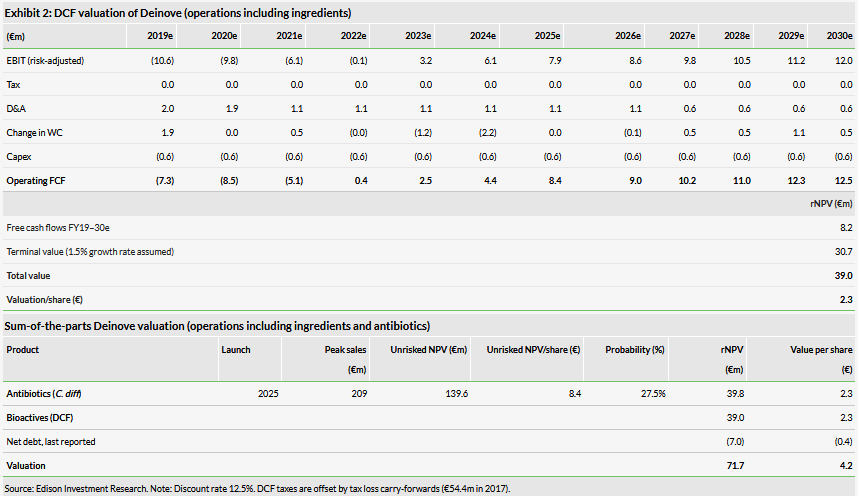
Valuation: €72m or €4.2/share
Our risk-adjusted NPV is slightly higher at €72m or €4.2/share from €66m or €4.2/share previously, mainly due to rolling our model forward. Deinove’s H119 results were largely in line with our expectations and we have therefore made no change to our estimates. As of end of August 2019, Deinove’s cash position was c €3.9m (excluding government debt). In July 2019, Deinove issued convertible notes totalling up to €15m, which could provide funding until the end of Q121.
Business description
Deinove is a biotechnology company that discovers, develops and produces high value-added compounds using its state-of-the-art bacterial strain selection, banking, fermentation and screening facilities. The most valuable compounds in the pipeline are novel antimicrobials, with lead asset DNV3837 ready for the Phase II trial. Products for other applications, such as cosmetics and nutrition, will support drug development efforts.
Antibiotics – Phase II with DNV3837 for C. diff
Antibiotics to treat Clostridioides difficile (C. diff) are mainly given orally (metronidazole could also be given intravenously as a last resort, but that would be off-label use). This means that effective antibiotic treatment of severe C. diff is still lacking as oral treatments can struggle to reach the intestines because of a patient's pathological condition, while currently available intravenous antibiotics generally do not penetrate the gastrointestinal barrier and therefore do not reach the site of infection. DNV3837 is administered intravenously and designed to cross the gastrointestinal barrier unlike existing IV antibiotics and target the gut, the site of the infection, more effectively than orally administered antibiotics.
There are two intravenous antibiotics administered against severe C. diff: metronidazole and tigecycline. Metronidazole is mostly used orally and, due to increasing resistance, is no longer recommended as front-line therapy, while no controlled trials with tigecycline have been conducted in C. diff infections (IDSA guidelines). The lack of a regulatory approved intravenous treatment option and growing incidence of C. diff present a significant opportunity for DNV3837, in our view.
In our last outlook report, we presented a detailed analysis of the unmet need in C. diff treatment and the potential of DNV3837. The Phase II study, which is about to start, should last for around 10–12 months. The results will be a substantial catalyst for the share price. If positive, the next step would likely be a registration Phase III trial. Since DNV3837 is a novel antibiotic, it has obtained a QIDP (Qualified Infectious Disease Product) designation and Fast Track status from the FDA, which will significantly facilitate regulatory interactions.
US Department of Defense evaluated Deinove’s antibacterial compounds
In May 2019, Deinove announced that researchers at the US Army Medical Research Institute of Infectious Diseases (the US Department of Defense) evaluated Deinove’s DNV3681 (pharmacologically active drug derived from the DNV3837 prodrug) against Bacillus anthracis and Francisella tularensis. The US classifies the two pathogens as tier 1 biological threat bacteria. The standard treatment is ciprofloxacin (broad-spectrum fluoroquinolone), which is a widely used antibiotic for various other infections and therefore at risk of decreasing effectiveness due to increasing bacterial resistance. The US researchers concluded that DNV3681 was more effective than the comparator antibiotic (ciprofloxacin) against Bacillus anthracis and recommended further testing in animal models (further steps not yet confirmed by Deinove). The findings were presented at the annual congress of the American Society of Microbiology in June 2019.
Integration of CRISPR-cas9 technology to accelerate antibiotic discovery
In September 2019, Deinove announced the expansion of its technological platform by adding CRISPR-cas9 gene editing capability. Clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 or CRISPR-cas9 has revolutionised gene editing, as it is faster, cheaper and more accurate than older generations of gene editing solutions.
One of the key aspects of Deinove’s competitive advantage is the genetic engineering platform, which allows the optimisation of strains to produce desired compounds with various modifications. Examples include Deinococci bacteria engineered by Deinove to produce pure carotenoids on an industrial scale (Phyt-N-Resist) or manipulating Streptomyces to produce a pharmaceutical component initially produced by Microbacterium arborescens (Deinove’s DNB101/102 antibiotic programme). The addition of the new-generation gene editing tool CRISPR-cas9 is part of the so-called AGIR programme (which Deinove is part of, as we described in our last outlook). AGIR is supported by public funds and will allow the identification and optimisation of new antibiotic structures to be accelerated, which should ‘feed’ the R&D pipeline.
Bioactives – new collaboration agreement with Dow
Deinove’s H119 report also provided an update on its bioactives portfolio. The aim of Deinove's bioactives programme is to provide a range of naturally produced, alternative, quality, sustainable and cost-effective bioactives for various industries. The two most-explored directions so far are in the cosmetics and animal nutrition sectors. We reviewed these opportunities in our last published outlook report. Recent new developments include:
The first product, launched in April 2018, is Phyt-N-Resist (phytoene), a 100% pure colourless carotenoid that cannot be extracted in pure form from vegetable sources. Carotenoids are known for their antioxidant and anti-ageing properties. Univar is taking care of the commercial promotion and distribution of Phyt-N-Resist in the EMEA zone, while Solvay (BR:SOLB) Novecare is marketing it in North America and Asia. Since the launch, Deinove has conducted preclinical studies to clarify the mechanism of action of its phytoene. The results showed that phytoene stimulates laminin production which, like collagen, is a major structural protein in the basal layer (lamina) of the skin (the junction between the epidermis and the dermis). Laminin has been identified as a target for dermatocosmetic applications to promote cellular regeneration and prevent ageing. This updated understanding about the mechanism of action of Deinove’s phytoene was presented by Solvay at the New York Society of Cosmetic Chemists (NYSCC) Suppliers' Day in May 2019. So far, no sales have been booked, but Deinove indicated that ‘several dozens’ of potential customers have been evaluating samples and the first sales could start by end of this year.
Hebelys was the second product launched, which was the result of a collaboration with Greentech, a French company focused on developing active ingredients from plants, algae, micro-algae and micro-organisms for application in the cosmetic, nutraceutical and pharmaceutical sectors. Hebelys is an anti-ageing cosmetic ingredient obtained through fermentation of the Sphingomonas hydrophobicum bacteria. In September 2019, Deinove announced the publication of a peer-reviewed article in the International Journal of Cosmetic Science. The article summarised results from an in vitro test (on artificial skin) and a clinical study (24 women included) conducted jointly by Deinove and Greentech. The studies evaluated the effect of Sphingomonas hydrophobicum extract (Hebelys) on cell senescence and skin structure, and showed that Hebelys delays skin ageing by slowing cellular senescence and restructuring the skin. This translated into improved well-being endpoints in the clinical trial. As with Phyt-N-Resist, such data support certain differentiated marketing claims, which is typically a key strategic element in commercialising cosmetic ingredients. Deinove indicated that several cosmetic brands have already confirmed their interest and ordered samples to integrate into their brand formulations.
In June 2019, Deinove announced a collaboration agreement with Dow to develop a new cosmetic ingredient, which selected one extract from Deinove’s proprietary bacterial bank. While no further details about the ingredient have been provided, the collaboration is another sign of external validation of Deinove’s expertise in bacterial extracts by a large multinational company. Deinove is responsible for optimisation of the production process, while Dow will be responsible for integrating the ingredient into its portfolio and will have worldwide market exclusivity. The commercialisation is expected to begin in early 2021.
Deinove has also reported progress with its animal feed collaboration, COLOR2B, with Avril, a French agro-industrial group. The partners validated the ingredient for animal feed, which will be positioned as a nutrient of biological origin versus other currently available petrochemical products. The product will be marketed as raw material for animal feed. The next steps include scaling up the manufacturing by the end of this year and the partners expect to start marketing the first ingredient by end of 2020.
Financials
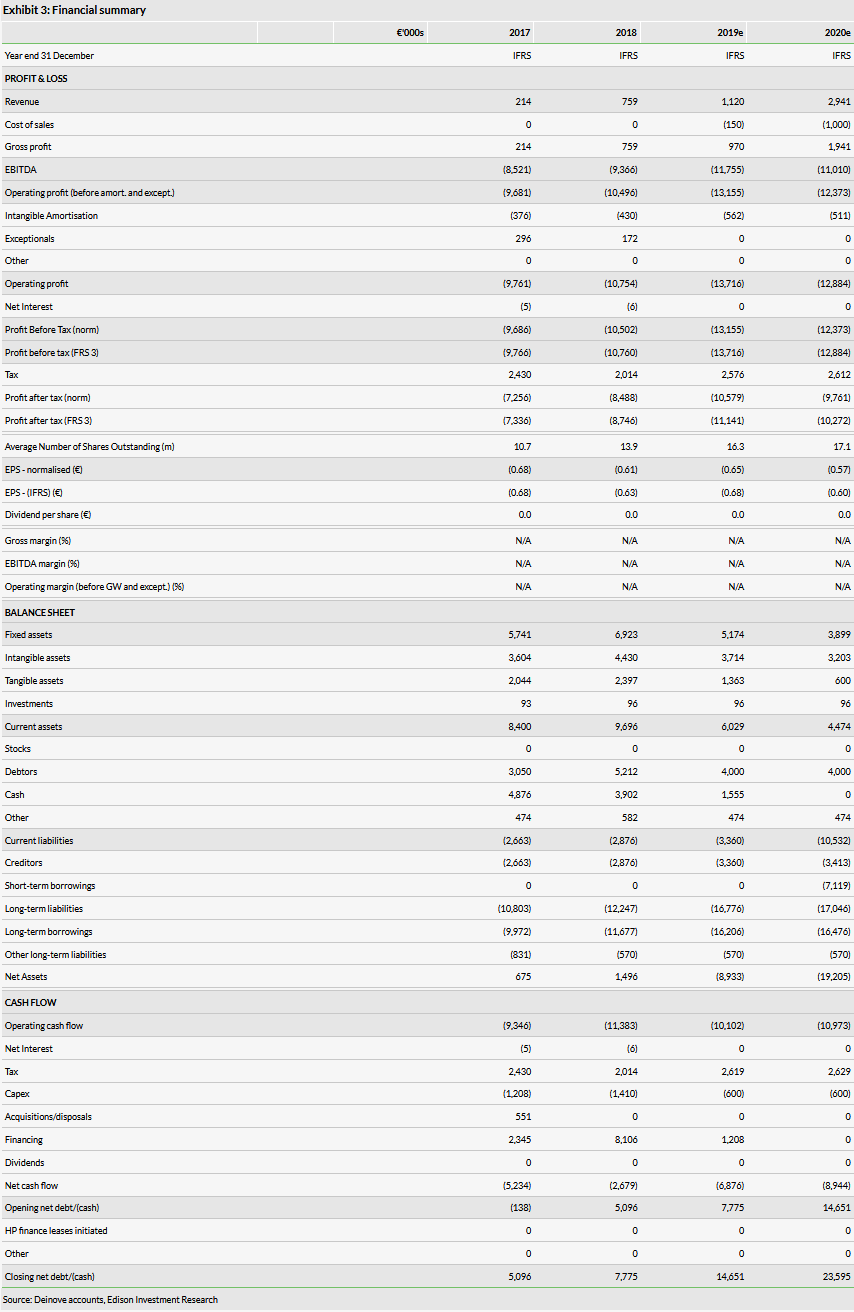
In H119, Deinove reported operating revenue of €405k, which was mainly a government grant from Bpifrance under the AGIR programme. Total operating costs amounted to €6.6m, of which €5.4m was R&D related. The year-on-year increase in operating expense is mainly due to higher R&D costs (+30%), which is a result of the ongoing Phase II trial with DNV3837. H119 R&D tax credits were €1.1m. As Deinove’s H119 results were largely in line with our expectations, we maintain our FY19 and FY20 revenue forecasts of €1.12m and €2.94m respectively, which mainly include grant payments and expected initial sales of cosmetic products. Our FY19 and FY20 operating loss estimates also stay at €13.7m and €12.9m respectively.
Deinove reported cash of €1.9m at the end of H119 and had €12.7m booked as long-term debt (€12.2m is conditional government loans, which are repayable if the products achieve commercial success).
In July 2019, Deinove entered into an agreement with the European Select Growth Opportunities Fund to issue a two-year convertible bond with a maximum nominal amount of €15m, 6.5% discount and interest free. The first tranche of €2.2m was issued at the same time the agreement was signed. Deinove indicated that funding like this provides financial visibility for the next 12 months (until end Q121) based on current operating plans. The Phase II readout is expected sometime next year, which represents an opportunity for significant value inflection.
In conjunction with the convertible debt funding, Deinove suspended its existing equity financing line with Kepler Cheuvreux (in H119 Deinove raised €1.1m from this set-up). In August 2019, it received €1.6m (net) from Société Générale (PA:SOGN) Factoring as partial pre-financing of the 2018 R&D Tax Credit (CIR).
Valuation
Our risk-adjusted NPV is slightly higher at €72m or €4.2/share versus €66m or €4.2/share previously, mainly due to rolling our model forward. However, our valuation per share has not changed due to the dilutive effect of the newly issued shares. We maintain all our assumptions for the R&D projects, as described in our outlook report.
We note that as of end-H119 there were unexercised share warrants and options amounting to c 10.7m; however, an absolute majority (8.0m) of those were related to the acquisition of Biovertis/Morphochem. The warrants associated with the latter become exercisable only after certain milestones are met, which are related to clinical development. Each of these milestones is related to positive R&D developments, which typically provide catalysts for the share price.