Clal Biotechnology Industries Ltd (TASE:CBI) recently published its first quarter update. Notably, MediWound has licensed North American commercialisation rights for NexoBrid to Vericel for $17.5m upfront, with an additional $132.5m in potential milestones. Also, Gamida Cell is on track to complete enrolment for its Phase III trial of NiCord (now called omidubicel) in haematological malignancies in H219 with data expected in H120. In addition, Cadent received a $15m milestone payment from Novartis as MIJ821 (CAD-9271) for treatment-resistant depression entered Phase II.
MediWound licenses NexoBrid
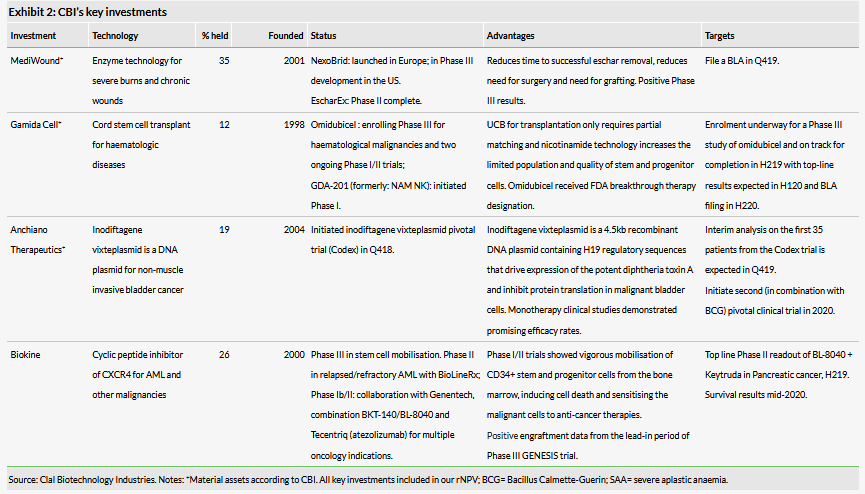
In May, MediWound (35% owned by CBI) announced it has licensed the North American commercialisation rights of NexoBrid to Vericel for $17.5m upfront and a total of $132.5m in potential milestones including $7.5m upon FDA approval. Royalties will range from single digits to low double digits (we are modelling 8–12% royalties). The company expects to file for approval in Q419. MediWound also announced that it will continue to seek partners outside of North America for NexoBrid, which could provide some additional funding.
BARDA commits an additional $21m for NexoBrid
Also in May, MediWound announced that the US Biomedical Advanced Research and Development Authority (BARDA) upsized its awarded contract with the company by $21m to initiate a NexoBrid expanded access treatment protocol (NEXT) in burn patients. Total non-dilutive funding from BARDA can now total up to $196m, with $31m provided as of the end of Q119.
Gamida Cell Phase III data next year
Gamida Cell (12% owned by CBI) is on track to complete enrolment for its Phase III trial of NiCord (now called omidubicel) in haematological malignancies in H219 with data expected in H120. If these Phase III data are positive, Gamida Cell plans to submit a biologic licence application (BLA) filing for omidubicel in H220.
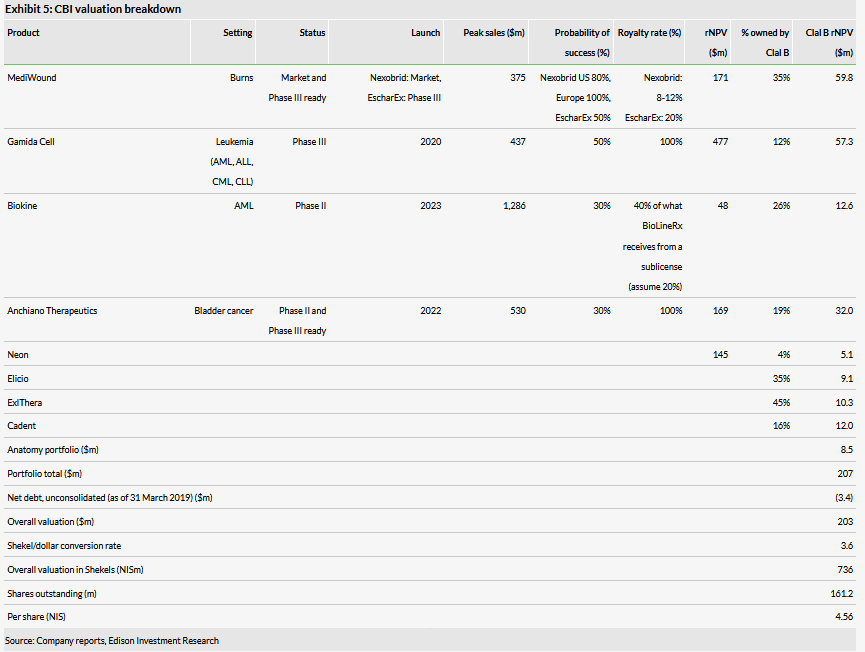
Valuation: NIS736m or NIS4.56 per share
We have decreased our valuation of CBI from NIS850m or NIS5.27 per share to NIS736m or NIS4.56 per share, primarily as a result of adjusting our NexoBrid model for the terms of the licensing deal. We have also lowered the value of the Neon asset due to its recent stock performance.
Business description
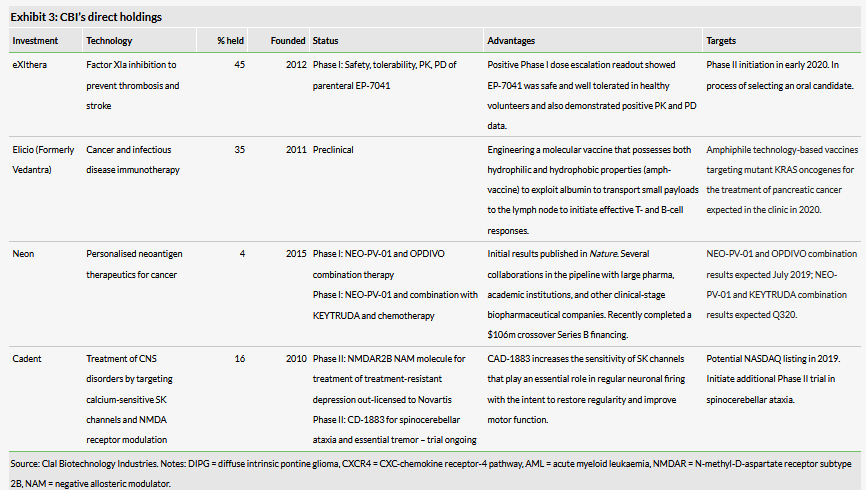
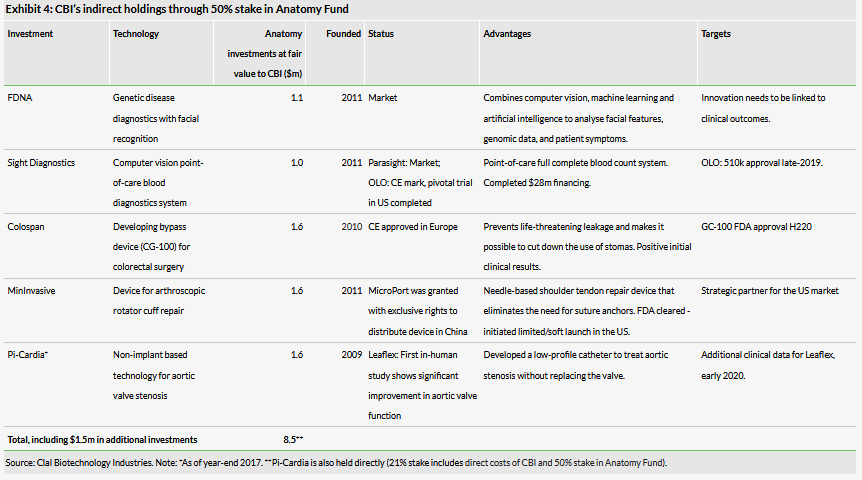
Clal Biotechnology Industries is a healthcare investment company focused on investing in a variety of therapeutic, diagnostic and medical device companies covering a full range of development phases from preclinical to post-market. The company holds nine direct investments, with interests ranging between 4% and 54%. It also has five indirect investments through its 50% stake in the Anatomy Fund, which it manages.
NexoBrid licence deal
In May, MediWound announced it has licensed the North American commercialisation rights of NexoBrid to Vericel, which manufactures and commercialises advanced cell therapies for the sports medicine and severe burn care markets. Vericel (Nasdaq: VCEL) markets an autologous cellularised scaffold product indicated for the repair of symptomatic, single or multiple full-thickness cartilage defects of the knee with or without bone involvement in adults and a permanent skin replacement for the treatment of patients with deep dermal or full thickness burns. Total net sales for both products were $90.9m in 2018.
The terms of the agreement are for a $17.5m upfront and a total of $132.5m in potential milestones including $7.5m upon FDA approval and $125m in sales-related milestones to be paid by Vericel to MediWound. The first sales milestone of $7.5m will be triggered at an annual net sales level in North America of $75m, a relatively high threshold as we model $91m in peak sales for the product in the United States. Royalties will range from single digits to low double digits (we are modelling 8–12% royalties), which we view as relatively low for a product with positive Phase III data. The company also entered into a supply agreement with MediWound where Vericel will pay MediWound to manufacture NexoBrid on a cost plus fixed percentage basis. In addition, as BARDA has committed to procure NexoBrid (pending FDA authorisation), Vericel will pay a percentage of gross profits on initial committed amounts and a royalty on any additional BARDA purchases of NexoBrid beyond the initial committed amount.
With regards to BARDA, in late May, the organisation upsized its awarded contract with MediWound by $21m to initiate a NexoBrid expanded access treatment protocol (NEXT) in burn patients. Total non-dilutive funding from BARDA can now total up to $196m (with $31m provided so far) and has several components. BARDA is providing technical assistance and a total amount of $77m (which includes the $21m that was just announced) in funding for NexoBrid development activities towards FDA approval. BARDA also maintains $16.5m for the procurement of NexoBrid contingent on FDA emergency use authorisation and/or FDA marketing authorisation, a $10m option to fund development of other potential NexoBrid indications and an option to fund up to $50m for additional NexoBrid procurement. BARDA is also supporting the development of NexoBrid as a debridement product to treat sulfur mustard injuries, providing $12m in funding to support research and development activities up to pivotal studies in animals with options for additional funding of up to $31m for additional development activities through BLA submission to the FDA.
As a reminder, on 22 January 2019, MediWound announced positive top-line results from its US NexoBrid Phase III (DETECT) trial at 44 burn centres. 175 patients with deep partial thickness (DPT) and full thickness (FT) thermal burns were randomised to receive either NexoBrid, SOC or gel vehicle (placebo) at a ratio of 3:3:1, respectively. The study achieved the primary endpoint, which was incidence of complete debridement, with statistical significance, as well as several secondary endpoints (Exhibit 1). Additionally, the trial reached its safety endpoint with statistical significance, which was non-inferior time to complete wound closure with NexoBrid versus SOC.
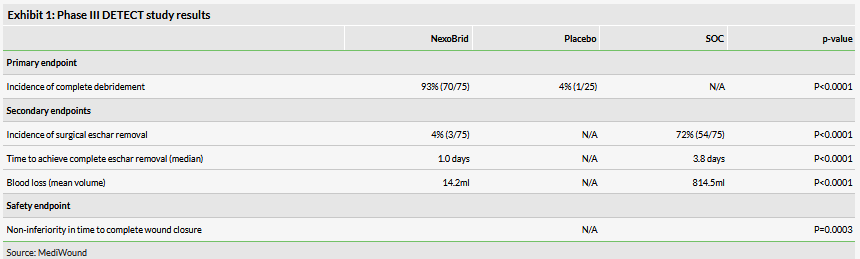
MediWound plans to have a pre-BLA meeting with the FDA to request submission of the BLA based on these acute primary, secondary and safety data and then further supplement the application with 12-month follow-up data during FDA review. If the meeting goes according to plan, the company expects to file a BLA in Q419, which would imply an approval in H220. However, if the FDA does not permit BLA submission with only acute data, the company expects its timelines to be delayed by about three to four quarters (to allow the firm to collect and submit 12-month data).
Also, MediWound reported Q119 results. Revenues, which are based on NexoBrid sales in the EU, were $0.5m, down 11% from Q118. The company is working on accelerating these sales and we expect it to announce a plan around the next earnings call. The company reported a post-tax loss of $4.1m in Q119, which is down 10% from the same quarter a year ago. As of 31 March 2019, the company had $21.5m in cash (including equivalents and short-term deposits) which subsequent to quarter-end was increased by the $17.5m upfront from the licensing agreement. According to the company, it plans to concentrate these resources on progressing the EscharEx development programme while NexoBrid becomes a self-funded product via BARDA backing.
Gamida Cell Phase III to complete enrolment in H219
Gamida Cell’s 120-patient Phase III study of omidubicel (formerly NiCord) in patients with haematological malignancies is ongoing. Omidubicel, which is the company’s lead asset, expands umbilical cord blood (UCB) cell graft ex vivo and enriches the specific subpopulation of stem and progenitor cells to treat haematological malignancies such as leukaemia and lymphoma. Essentially, CD133+ cells selected from a single unit of UCB are cultured for 21 days in nicotinamide resulting in a c 100-fold expansion of dose stem and progenitor cells, which are then cryopreserved until they are transplanted into the intended patients. This expansion is expected to provide a substantial advantage over a single UCB graft. The use of UCB for bone marrow transplantation (BMT) is limited by the minimal number of stem and progenitor cells. The omidubicel process seeks to provide a more viable alternative to BMT in cancer patients and only partial genetic matching is needed (ie a minimum requirement of four out of six human leukocyte antigen biomarkers). The registrational trial is investigating the ability of omidubicel to provide a graft with an ample number of cells that have fast and vigorous in vivo neutrophil- and platelet-producing potential to improve transplantation outcomes (as low cell dose is associated with delayed engraftment and poor outcomes). The primary endpoint for the trial is time to neutrophil engraftment following transplantation (on or before the 42nd day post-transplant) compared to a non-manipulated cord blood unit. Enrolment is on track for completion in H219 with top-line data expected in H120. Provided that these Phase III data are positive, Gamida Cell plans to submit a BLA filing for omidubicel for the treatment of haematological malignancies in H220.
As a reminder, in data from a Phase I/II trial from 36 evaluable patients with acute leukaemia, myelodysplastic syndrome (MDS) and lymphoid malignancies (most recently presented at the International Society for Cell and Gene Therapy [ISCT] 2019 Annual Meeting in May), omidubicel demonstrated a median time to neutrophil engraftment of 11.5 days and a median time to platelet engraftment of 34 days. According to case-matched data from the Center for International Blood and Marrow Transplant Research, standard UCB treatment results in a median time to neutrophil engraftment of 21 days and a median time to platelet engraftment of 46 days. These data indicate that omidubicel has the potential to be the graft of choice for patients without a matched donor.
The company ended Q119 with $50.3m in cash and has guided for $35–40m in cash outflow for operating activities over 2019, which should be able to fund the company until around when the Phase III data are expected in H120.
Cadent receives milestone from Novartis
Cadent (16% owned by CBI) announced in May that it had received a $15m milestone payment from Novartis as MIJ821 (CAD-9271) for treatment-resistant depression entered Phase II. Cadent and Novartis entered into a licence and collaboration agreement in 2015 to advance their subtype selective negative allosteric NR2B-containing N-Methyl-D-aspartate (NMDA) receptor modulators to treat depression. NMDA receptors are glutamate-gated ion channels and are critical to central nervous system development, the production of rhythms for breathing and locomotion, and underlying synaptic plasticity, cognition and memory.1 Hyperactivity or hypofunction of the NMDA receptor system, which is the primary receptor for all excitatory neurotransmission that exists as multiple subunits with distinct properties, contributes to nervous system disorder pathophysiology such as depression, schizophrenia, pain and chronic neurodegenerative diseases.2
Under the financial terms of the agreement with Novartis, Cadent received a $6m upfront payment and is entitled to receive up to a total of $180m for development milestones, up to a total of $200m for sales milestones and royalties of up to 10% of total annual sales.
Cadent also announced that it received orphan drug designation from the FDA for CAD-1883 in spinocerebellar ataxia. CAD-1883 is a positive allosteric modulator of calcium-sensitive potassium (SK) channels. CAD-1883 increases the sensitivity of SK channels, which play an essential role in regular cerebellar neuronal firing, with the intent to restore regularity and improve motor function for the potential treatment of spinocerebellar ataxia, an orphan genetic disorder characterised by cerebellum dysfunction or degeneration that causes difficulty coordinating movements, and essential tremor, a neurological disorder characterised by involuntary and rhythmic shaking, most commonly of the hands and forearms. It is estimated to affect 11,000 people in the US and an additional 15,500 people in the EU5 and Japan. The company intends to initiate two Phase II trials in essential tremor and spinocerebellar ataxia.
Updates from other investments
Sight Diagnostics (owned by CBI via its 50% stake in the Anatomy Fund), which is developing a point-of-care full complete blood count (CBC) system, announced in April that it had completed its pivotal trial. According to clinicaltrials.gov, the trial had 700 participants across five sites in Israel and the US.
Pi-Cardia (21% held by CBI through direct investment and its 50% stake in the Anatomy Fund) announced in May the successful completion of the first in-human study for its Leaflex Performer catheter. Leaflex is a low-profile catheter intended to treat aortic stenosis without replacing the valve and can be implanted in a procedure that takes less than 20 minutes. The study was in 16 transfermoral cases and demonstrated that the use of Leaflex was both safe and feasible. Hemodynamic improvement was reported to be significant and greater than previously reported with balloon valvuloplasty. The company announced it will be moving Leaflex forward in clinical development.
Valuation
We have decreased our valuation of CBI from NIS850m or NIS5.27 per share to NIS736m or NIS4.56 per share, primarily as a result of adjusting our NexoBrid model for the terms of the licensing deal (we had previously modelled as if it was going to market NexoBrid itself, and assumed an NPV neutral licensing deal). The upfront payment was of a decent size ($17.5m), but the $125m in sales milestones only start being paid out once sales hit $75m in North America (with a $7.5m payment), which leads us to believe few of those will be paid out as our peak annual sales estimate in the region is $91m. Also, we would have expected a larger royalty rate for a product with positive Phase III trial data. The company disclosed tiered royalties that range from single digits to low double digits and we model 8–12%. Our sales estimates for NexoBrid are unchanged.
We have also lowered the value of the Neon asset due to the recent stock performance as that valuation is based on the value of the publicly traded shares.
Financials
Due to significant ownership stakes, CBI consolidates the financials of several of its investments (MediWound, CureTech and the Anatomy Fund) and, on this basis, it had NIS94.7m in cash, cash equivalents and bank deposits as of 31 March 2019. CBI’s cash position at the corporate level (excluding consolidation) was NIS13.3m at the end of the quarter, with NIS25.6m in debt attributed to loans from a controlling shareholder (due in 2025).
Total consolidated revenues of NIS1.8m in the quarter were primarily generated through the sales of MediWound’s NexoBrid in Europe, Israel and Argentina for the year, which is down roughly 21% from Q118. The company also reported NIS16.1m in revenue as a realised gain from the decrease in equity interest of associates during the quarter.
Total consolidated R&D spend was NIS4.7m for the quarter, down 38% compared to the same quarter last year. General and administrative costs, which include payroll and related expenses, management fees, and marketing and advertising expenses on a consolidated basis were NIS13.8m, down 21% compared to Q118.
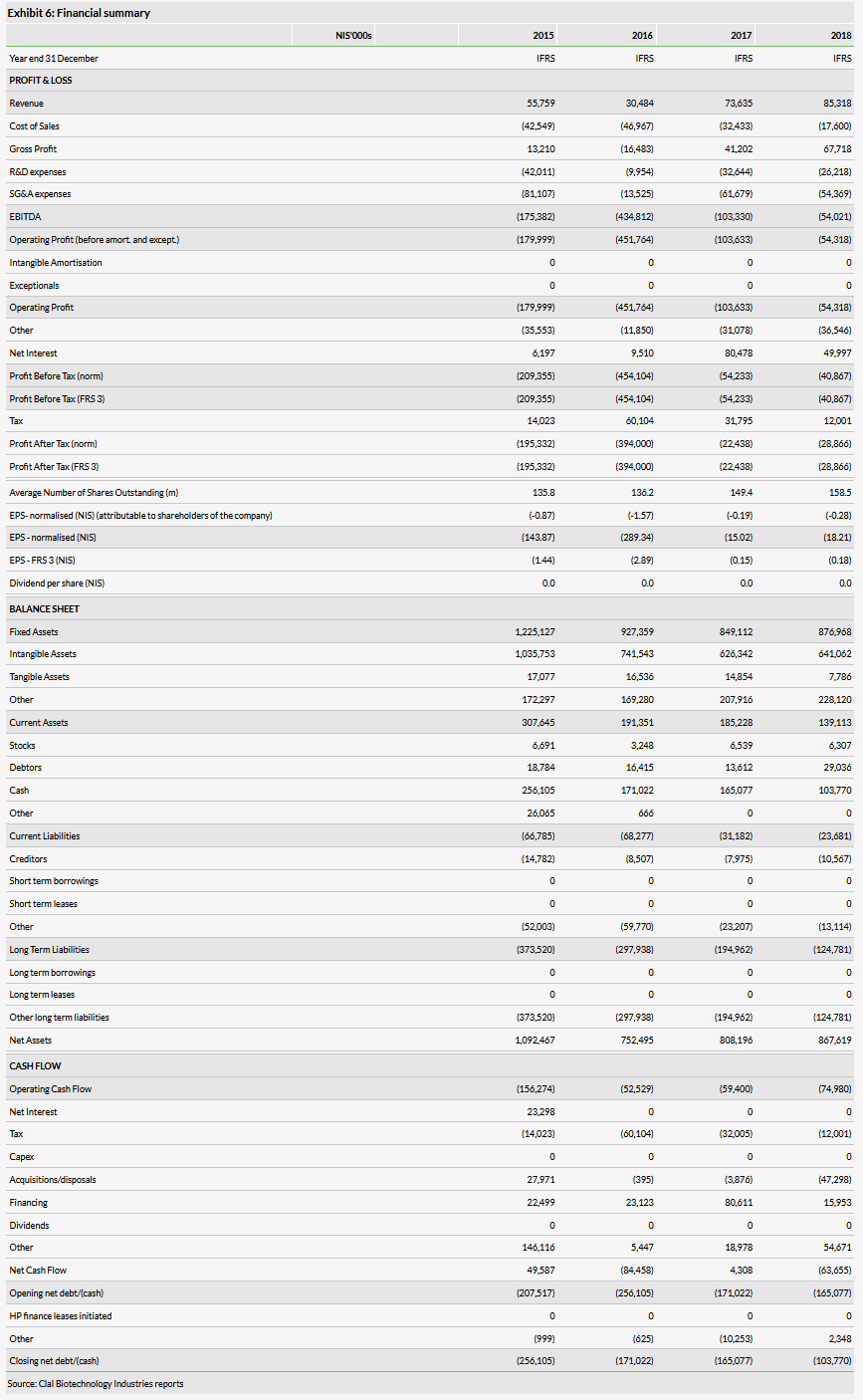
We outline historical financials in Exhibit 6; however, we are not providing forecasts at this time.