Steady progress
FY13 results show year end cash of €22.1m. This will fund the current CHART-1 Phase III for cardiac regeneration and last throughout 2015. CHART-1 recruitment is stated to be on target to provide data by late 2015. C-Cure autologous stem cells are a potentially powerful novel treatment for patients with weakened, scarred hearts. C-Cure showed positive cardiac efficacy endpoints in Phase II and median heart output increased by 25% relative to baseline. The FDA has approved the part US-based CHART-2 design. The value remains at €77 per share.
Phase III: CHART-1 (EU) and CHART-2 (US-EU)
CHART-1 is a European Phase III trial with a hierarchical outcome score focused on mortality and morbidity. Patients will be in, or recovering from, late-stage (NYHA Class III and IV) heart failure and only pumping half or less of the normal amount of blood. Cardio3’s C-Cathez catheter is used to increase the retention of cells in the heart to around 36%, a 3.6 fold gain. Top-line data are due in late 2015 with EMA regulatory filings possible from 2016. Management has stated that recruitment is on target but numbers have not been released. If C-Cathez is FDA-approved, CHART-2 could also use C-Cathez, possibly improving its probability of success. CHART-2 has a six-minute walk primary endpoint with a 40 metre improvement threshold. CHART-2 may be partnered in the US to gain trial funding with a possible 2017-18 filing. The FDA agreed the CHART-2 design in January 2014. The C-CURE Phase II showed that C-Cure was safe and showed a 25% median relative increase in cardiac output. This enabled patients to walk further in the six-minute walk test.
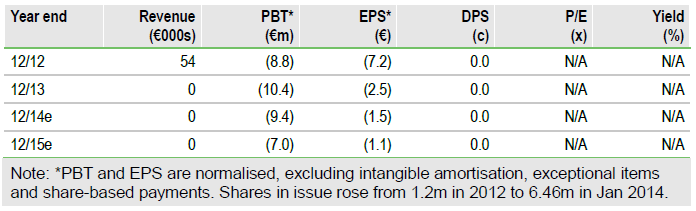
FY13 results: Strong cash, refocused preclinical
Cardio3 had December 2013 cash of €22.1m with €2.5m of Walloon cash funding due to 2015 and €1.5m of repayment rescheduling. Manufacturing and clinical costs were €6.9m in total. R&D was €2.6m (including capitalised costs). The portfolio now focuses on GQR-4, an antibody to prevent cardiac reperfusion injury on acute treatment. Admin costs were €3.0m. Cash outflow before financing was €11.2m.
Valuation: Huge market, huge need, huge potential
Cardio3 may have a customised therapy for about a million moderate to severe heart failure patients in North America and the EU. The main Cardio3 market is the EU with the US being partnered. Total end-user sales could be €1.9bn with no immediate regenerative therapy competitors. Based on clinical probabilities of EU 40% and US 25%, the base case indicative value remains at €77 per share. Scenario risk variants range from €42 to €225 per share.
To Read the Entire Report Please Click on the pdf File Below