BioPorto (CO:BIOPOR) has reported revenue of DKK26.0m for 2018. The company’s main strategic objective is the development and approval of The NGAL Test, but its ongoing sales of research products provide insight into future markets for the test. In particular, the company reported DKK9.2m in sales of the test for research purposes, an increase of 41% globally and 80% in the US. This demonstrates increased traction and appreciation of NGAL as an important biomarker for acute kidney injury (AKI).
Laying the groundwork for the launch
The advancement of the research-use only NGAL Test is important beyond the direct revenue it provides by increasing the exposure of physicians to the technology and providing a base of customers that can be leveraged after the launch of the clinical version of the test. We consider the increasing utilisation of the test in research settings is an encouraging indicator of the potential market for the product. The company has noted that growth in the US was driven by increased adoption at hospitals.
Submit paediatric application in H1, adult in H2
BioPorto provided an update on the timelines for its ongoing development programmes in its annual report. It is finalising an application package for its paediatric AKI application, which it expects to file in H119. Following previous FDA feedback, the company has designed a study to support its adult AKI application, which is planned to initiate in Q119 and enrol 150–200 patients. The company plans to submit an adult AKI application in H219.
Other products slow
The sales of both ELISA kits and antibodies were slower in 2018 than previous periods. The company attributed this to market forces leading to fewer large bulk orders of product. Our outlook for these products is flat as we expect most of the revenue growth and sales efforts to be driven by The NGAL Test.
Valuation: Increased to DKK952.8m
We have increased our valuation to DKK952.8m from DKK895.0m, although it is flat on a per-share basis (DKK5.75 from DKK5.76 per basic share). This is driven by rolling forward our NPVs and higher net cash following the November private placement (10.2m shares for DKK40.0m) and is offset by higher administrative and other unallocated costs and lower valuation for research products. We expect the company will require an additional DKK35m in cash before profitability in 2021.
Business description
BioPorto is a diagnostic company focused on the development and marketing of antibodies and other products for research and diagnostics. This includes a portfolio of products marketed for research use and The NGAL Test for predicting acute kidney injury. Two 510(k)s will be submitted to the FDA for clearance of The NGAL Test in 2019.
Commercial and financial update
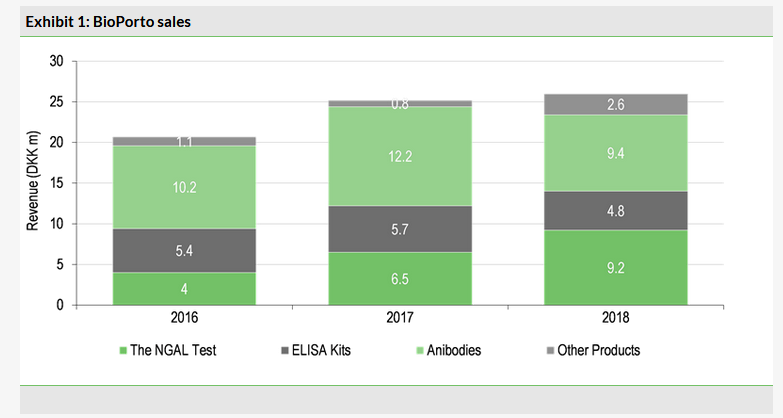
BioPorto’s revenue of DKK26.0m represents a slight increase over 2017 (DKK25.2m). This is slower than the increase in previous years (2016: DKK20.7m). The company has an increasing shift in focus and resources toward the development and promotion of The NGAL Test. These efforts have translated into a continued increase in sales of The NGAL Test for research: DKK9.2m in 2018, a 41% increase over 2017 (DKK6.5m). In particular, BioPorto stated that sales of The NGAL Test were up 80% year on year in the US (DKK4.5m from DKK2.5m), the primary territory the company is targeting for approval of the clinical version of the test. More important than the revenue from these efforts is the increased awareness surrounding the test that these trends indicate. We expect utilisation in the research setting to increase awareness of the test before launch and provide an existing sales pipeline of doctors and hospitals to target. Testing for NGAL in the research setting can be used for a number of applications, but we expect the increased first-hand exposure to further the goal of establishing it as an important biomarker in the clinic and among key opinion leaders. The increase in US sales of the test is directly attributable to increased adoption in hospitals, which we find highly encouraging.
Sales of ELISA kits and antibodies were both down year on year (Exhibit 1), which the company attributed to fewer bulk orders. Given the continued focus on The NGAL Test, our outlooks for these products are flat for the near future.
The company has two ongoing clinical studies of The NGAL Test: a paediatric application for urine NGAL and an adult application using plasma NGAL. The applications will be submitted to the FDA in H1 and H219 respectively. The company may recognise a small revenue from the products in 2019 if it receives market clearance by YE19. However, we expect any near-term revenue to be offset by sales and administrative expenses associated with the launch (DKK51.5m total in 2019). This is increased from our previous estimates (DKK36.3m) to align with current spending trends and company guidance. We have increased our R&D expenditure for 2019 (DKK20.5m, from DKK10.0m previously) because we originally expected more of the costs of the ongoing clinical studies to be incurred in 2018, so these costs have been shifted to 2019. Following this change and an increase in our 2019 SG&A forecast, our 2019 EBITDA loss estimate increased from DKK17.7m to DKK40.7m. The company stated that the study to support the adult AKI application is planned for initiation in Q119. We expect R&D spending to further increase in 2020 (DKK36.6m) as the company expands The NGAL test into additional indications.
The company ended 2018 with DKK46.7m in cash following a private placement in November. It issued 10.2m shares at DKK3.93 per share for gross proceeds of DKK40.0m. We expect this cash to provide a runway into 2020 and anticipate the company will require DKK35m in additional cash to reach profitability in 2021. We record this financing as illustrative debt in 2020.
Valuation
We have increased our valuation to DKK952.8m from DKK895.0m, although it is flat on a per-share basis (DKK5.75 from DKK5.76 per basic share) due to the increased share count following the November 2018 offering. The increase in valuation is driven by rolling forward our NPVs and higher net cash and is offset by higher unallocated costs (DKK201.0m from DKK174.8m, previously) and a more tempered outlook for research products (DKK18.3m valuation from DKK33.1m, previously). The increase in unallocated costs is associated with adjustments to our overhead estimates following the recent report. We expect to update our valuation if the company reports the details from its clinical studies or interactions with the FDA.