BioPorto (CO:BIOPOR) announced that it had submitted an application to the FDA for the paediatric NGAL Test. The application was specifically for the risk assessment of acute kidney injury in children under the age of 21. The application was submitted with the breakthrough designation, which we consider very encouraging. The designation shortens the statutory review time to 45 days (from 90) and provides that a portion of data needed be provided after marketing clearance.
Sensitivity/specificity as expected
The application was made on the basis of a retrospective study of urine samples previously collected to test the NGAL ELISA assay in paediatrics. The company reported a sensitivity of 65.0% and a specificity of 81.8%, with a negative predictive value in this population, which we consider roughly equivalent to previous data reported in adults (albeit not directly comparable).
Breakthrough designation: An FDA endorsement
The breakthrough designation received for this program is important because as part of the evaluation the FDA deemed that the data provides a ‘reasonable expectation that the device could provide for a more effective…diagnosis’ than the standard of care. We consider this an endorsement from the agency, and encouraging regarding its final marketing decision on the product.
Potential to complete data with postmarketing study
The breakthrough designation is additionally important for this product because it provides for initial clearance of a medical device with a lower threshold of clinical data supported by subsequent postmarketing studies. The company is currently seeking clearance for the test using retrospective clinical data, which the agency is typically more reluctant to accept, but with the breakthrough designation, there is the potential to fill any gaps with postmarketing studies.
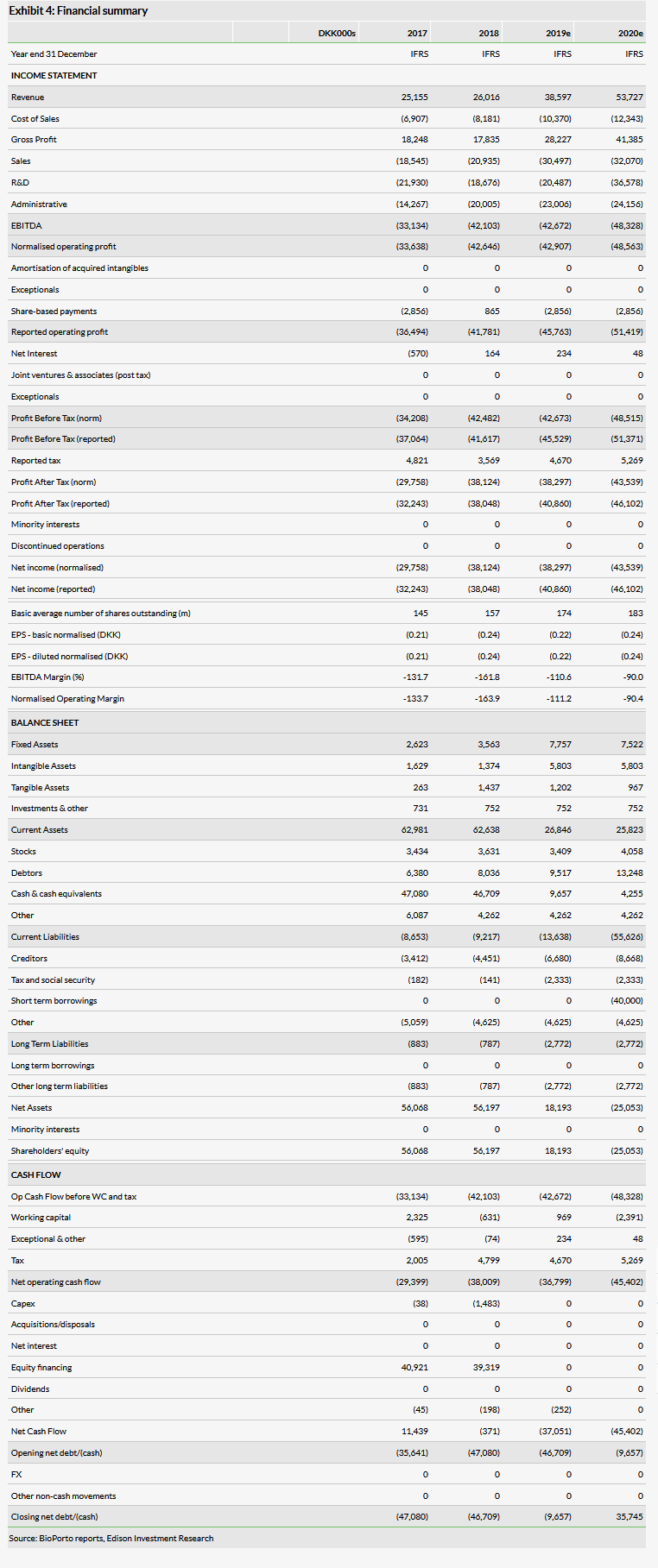
Valuation: Increased to DKK966m or DKK5.83/share
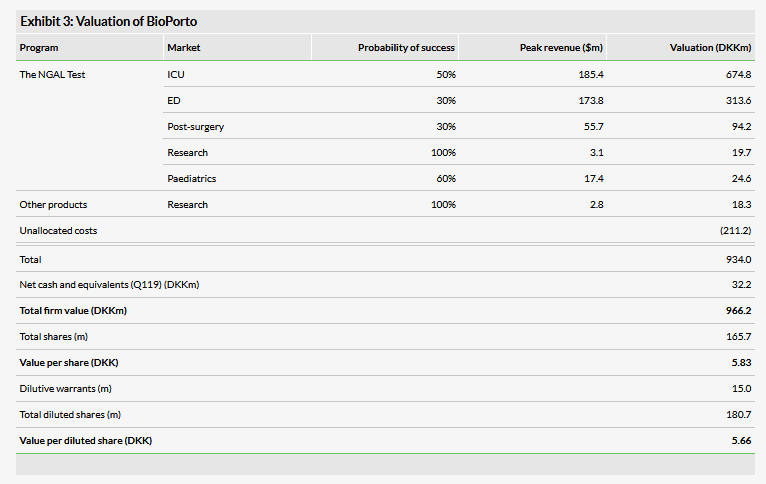
We have increased our valuation to DKK966m or DKK5.83 per share, from DKK952.8m or DKK5.75 per share. This is driven in part by increasing the probability of success for the paediatric NGAL test to 60% from 45%, based on the breakthrough designation, as well as rolling forward our NPVs. This is offset by an increase to administrative costs and lower cash (DKK32.2m).
Business description
BioPorto Diagnostics is a diagnostic company focused on the development and marketing of antibodies and other products for research and diagnostics. This includes a portfolio of products marketed for research use and the NGAL Test, for prediction of acute kidney injury. Two 510(k)s will be submitted to the FDA for clearance of the NGAL test in 2019.
Paediatric NGAL Test submitted
On 15 May 2019, BioPorto announced that it submitted an application with breakthrough device designation to the FDA for clearance of the paediatric NGAL Test. The NGAL Test is an assay to measure the presence of the NGAL biomarker in blood or urine for assessing the risk of acute kidney injury (AKI), and the current application is specifically for urine based NGAL in patients under 21.
The application was made on the basis of a retrospective study performed by the company using samples that were previously collected by the company from 32 paediatric ICUs. These samples were previously acquired for a study examining the NGAL ELISA assay, a different product also produced by the company. A total of 4,683 samples (of which 1,261 had AKI, 542 severe AKI) were collected for this previous study, of which an undisclosed subset were retested for the current study.
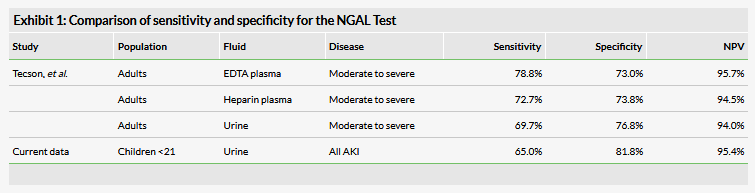
The company reported that the test had a sensitivity of 65.0% and a specificity of 81.8%, and an implied negative predictive value of 95.4% from this sample set. These data are roughly comparable to previously disclosed data on the NGAL Test reported in the literature (Exhibit 1).1 However, it is worth noting several key differences from these data, including that the prior study was performed exclusively in adults. Additionally, the data reported in Tecson et al. only examined the ability of NGAL to detect moderate to severe disease, whereas the current data includes mild (grade 1) disease. We consider this detail encouraging because mild disease detection has been problematic due to naturally lower NGAL concentrations, as well as difficulties confirming the diagnosis using serum creatinine (sCr), which can be inconsistent in these patients. An application for the NGAL Test was previously filed using the data from Tecson et al. and subsequently rejected ‘primarily because the dataset for mild cases of AKI did not support approval,’ according to the company. The inclusion of these patients in the current data suggests that it is less likely that mild disease patients will be cited as a deficiency in this application.
There are multiple benefits to the program. One benefit highlighted by management is a shortened statutory review time of 45 days (from 90), which should ensure a decision by the end of July. This timeline also includes increased communication with the agency, allowing the company to provide direct feedback on any outstanding issues during the review.
However, a very important benefit for this product in particular is that breakthrough designated devices may receive initial marketing authorisation with reduced clinical data requirements that are subsequently supplemented with postmarketing clinical data. This is important for the current application of the paediatric NGAL test because it is based on retrospective data. The agency is generally reluctant to authorize new devices based on retrospective data because they have a lower degree of statistical rigour than prospective studies. However, there are practical concerns around performing a prospective trial on paediatric AKI in the ICU. The number of clinical sites required and associated expense would be prohibitive. This is not uncommon for paediatric indications and the FDA has a mandate to facilitate reasonable accommodation in this area. We believe that if the test is cleared based on existing data, the FDA is likely to request a prospective postmarketing study, which should be substantially easier than a corresponding premarketing study. In general, we find the breakthrough designation highly encouraging regarding the potential clearance of this test.
The paediatric AKI testing market
As with other estimates of AKI prevalence, there has been significant variability in the rates of AKI in paediatric ICU populations. One of the most recent and comprehensive studies published in the New England Journal of Medicine found that 26.9% of patients admitted to 32 different paediatric ICUs had AKI. 3 11.6% of these patients had severe AKI, which was associated with a 77% increase in the risk of death, underlying the need for effective detection and intervention in this setting. However, the rate of AKI in children associated with hospital stays is small given the lower underlying rate of paediatric ICU admissions. Only an estimated 5% of ICU beds in the US are dedicated to paediatrics, or around 4,000 in total.4 We estimate approximately 240,000 admissions per year, based on reported occupancy of 59 patients per bed per year.5
Because of these factors, we believe that revenue from the paediatric market will be small compared to the much larger adult markets. Albeit, this is balanced with the low investment needed to date for this application. There are additional factors apart from revenue from paediatrics that we consider valuable. Clearance in paediatrics would allow a unified treatment algorithm where all ICU admissions are treated similarly. We believe this has the potential to aid adoption. Moreover, there is significant value in the goodwill in treating this population. Finally, there is the potential for clearance in paediatrics to translate into a future targeting of neonates. Neonates are hospitalised at significantly higher rates, accounting for 73% of all hospital admissions in patients under 18,6 and approximately 20,000 ICU beds.4 There is increasing awareness of AKI in this population and the associated risks: rates of AKI in very low birth weight infants have been measured as high as 40%.7
Valuation
We have increased our valuation to DKK966m or DKK5.83 per share, from DKK952.8m or DKK5.75 per share. We have increased the probability of success for the paediatric NGAL program to 60% (from 45%) on the basis of the recent submission with breakthrough therapy designation. We believe that the designation signals a willingness on the part of the FDA to consider clearance based on retrospective data, and that both this willingness and the lower clinical barriers are encouraging for the successful clearance of the product. Other adjustments to our model include rolling forward our NPVs, which is offset by lower cash (DKK32.2m vs DKK46.7m) and an increase to our unallocated costs associated with increased administrative costs going forward (more below). We expect to update our valuation following the clearance decision from the FDA for the paediatric test, expected by the end of July.
Financials
The company reported revenue of DKK5.5m and an operating loss of DKK17.1m for Q119. Revenue was down sequentially (from DKK8.9m in Q418), albeit roughly in line with previous first quarters (DKK5.7m in Q117, DKK4.6m in Q118). The company continues to expand its operations, including the hiring of a president of its US organisation. We have increased our administrative costs for 2019 and onward slightly (DKK23.0m from DKK21.0m) to account for building out the US apparatus. This has slightly increased our expected financing requirement to DKK40m from DKK35m, which we record as illustrative debt in 2020. The company ended the quarter with net cash of DKK32.2m. The company maintained guidance of approximately DKK 40m in revenue and an operating loss of approximately DKK 45m for the full year.