Multipotent stem cells
The near-term investment case for Athersys Inc, (ATHX) rests on the outcome of two Phase II studies of its novel, off-the-shelf, stem cell therapy product, MultiStem. Trial results in Q114 for ulcerative colitis (UC) and Q214 for ischaemic stroke have the potential to re-rate the stock and enhance the company’s options for fresh finance, although a newly secured two-year, $25m equity agreement with Aspire Capital provides financial flexibility. We value Athersys at $205m, or $3.40 per share, ahead of the key catalysts.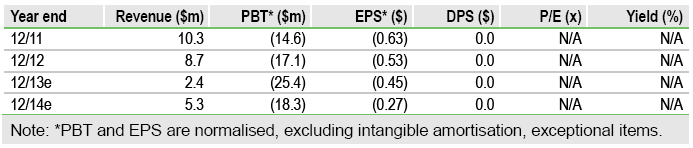
A novel and differentiated approach
Athersys is developing allogeneic (off-the-shelf), multipotent adult progenitor cells (MAPCs), derived from adult bone marrow. Similar to mesenchymal stem cells (MSCs), the company believes its MAPCs are substantially differentiated from MSCs and deliver enhanced anti-inflammatory effects. MAPCs are also highly proliferative, enabling the production of millions of clinical doses from a single adult donor. The cells can be frozen and stored for five years. The cell volumes can also be significantly higher than MSCs, with c 1bn cells administered in a single dose.
Approaching clinical validation
To date, two Phase I studies, in acute myocardial infarction (AMI) and graft-versus-host-disease (GvHD) have been completed with MultiStem. These trials provided encouraging signs of MultiStem’s safety and efficacy, but the outcome of the Phase II studies in UC and ischaemic stroke will go a long way to determining the validity of the MultiStem technology. A third Phase II trial, in AMI, is expected to start in mid-2014, supported by a $2.8m NIH grant, while the design of a Phase II/III study in GvHD is under discussion with the FDA.
Pfizer on board already
Pfizer has been a partner since 2009, for the use of MultiStem for inflammatory bowel disease (particularly UC and Crohn’s disease). Pfizer paid $6m upfront and the deal allows for a further $105m in milestones. Positive UC Phase II data could encourage Pfizer to conduct pivotal studies in UC and/or Crohn’s disease. The outcome of the stroke trial, a classical high-risk/high-reward indication, could also help attract new partners.
Valuation: $205m/$3.40 per share, ahead of catalysts
We value Athersys at $205m, or $3.40 per share, based on a sum-of-the-parts DCF valuation. This includes end-Q313 net cash of $18m. This represents upside to its market cap of $105m and $1.75 share price, ahead of the key catalysts in Phase II data for MultiStem in UC (Q114) and stroke (Q214). The new Aspire equity deal removes a financing overhang, but full draw-down could result in 20% dilution.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Athersys: Approaching Clinical Validation
Published 10/27/2013, 03:31 AM
Updated 07/09/2023, 06:31 AM
Athersys: Approaching Clinical Validation
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.