The European Medicines Agency (EMA) has adopted a positive opinion on J&J’s application for Dacogen (decitabine) for treatment of elderly patients with acute myeloid leukaemia (AML). The decision is the key hurdle to obtaining an EU-wide approval for the drug and thus represents a significant value-creating event for Astex. EU sales of Dacogen should ensure the continuation of a meaningful royalty stream to Astex after the expiry of the drug’s US exclusivity next year. It therefore puts Astex in a position to initiate planned new studies with AT13387 and SGI-110, without needing to raise new finance. This is the first of a number of catalysts for Astex expected in H212, which will also include the read-out from four Phase II studies. As a result of the CHMP decision, we value Astex at $560m.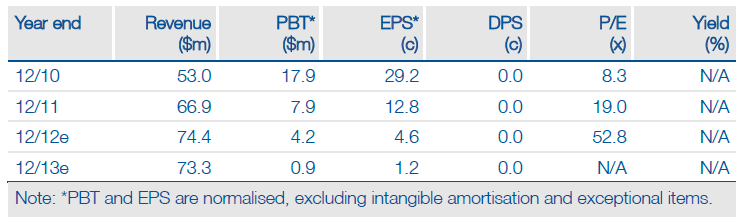
Positive Opinion Should Lead To Approval By September
The EMA’s positive opinion on Dacogen is now referred for formal approval by the European Commission, which is likely to come through by the end of September. This should pave the way for national approvals and launch, in some cases subject to pricing and reimbursement procedures, across Europe from 2013. The drug is to be indicated for the treatment of patients with newly diagnosed de novo or secondary AML who are not candidates for induction therapy (ie those over 65 years).
Substantial Royalties Confirmed Through 2023
Astex receives a substantial (c $65m per year) royalty on Dacogen and EU approval should ensure the continuation of a meaningful royalty stream after the expiry of the drug’s U.S. exclusivity next year, to the end of EU exclusivity in perhaps 2023.
Four Phase II Study Results Due This Year
Astex expects to see top-line data from Phase II studies with AT13387, SGI-110, amuvatinib and AT7519 this year. AT13387, an HSP 90 inhibitor, and SGI-110, a second generation hypomethylating agent, should each see two new Phase II study initiations this year: one will be in melanoma (AT13387) and one in ovarian cancer (SGI-110). Others indications have not been disclosed.
Valuation: rNPV Of $560m
We value Astex at $560m (including cash) on the basis of a presumed EU approval of Dacogen in AML, following the positive opinion from the CHMP. Dacogen contributes around half of the ex-cash figure.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Astex: EMA Issues 'Positive Opinion' On Dacogen
Published 07/27/2012, 12:03 PM
Updated 07/09/2023, 06:31 AM
Astex: EMA Issues 'Positive Opinion' On Dacogen
Astex Pharmaceuticals Positive Opinion On Dacogen
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.