Arrowhead’s investment case is evolving fast with the recent publication of early but impressive data on its anti-hepatitis B agent, ARC-520, in a chimpanzee model of chronic HBV infection. This showed a 90% reduction in hepatitis B antigens (HBeAg) and HBsAg, sufficient to hold out the potential that the agent could become a functional cure. With the initiation of a Phase I safety study of ARC-520 this year and Phase I/II efficacy study next year, Arrowhead is poised to have a major value inflection point once these data become available.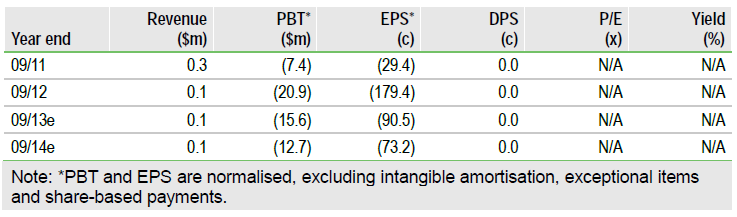
New ARC-520 Chimpanzee Data Are Impressive
Two injections of ARC-520 in a chimpanzee with chronic infection with HBV led to a 95% reduction in circulating viral DNA and 90% reductions in HBeAg and HBsAg. The reduction of these two antigens by ARC-520 raises the hope that the drug could become a functional cure of the disease, something that is achieved in less than 10% of patients by current standards of care.
Human PoC Data Could Come In 12-18 Months
Arrowhead is on track to start a Phase I safety trial in healthy volunteers in Q313 and a single-dose proof-of-concept pilot trial in HBV patients by the end of 2013 or early 2014. A multiple-dose Phase II trial should start in H214.
ARC-520 Will Become The Main Value Driver
Despite its relatively early stage of development, ARC-520 is set to become Arrowhead’s most important drug candidate and value driver since it addresses a multi-billion market in HBV and has shown profound efficacy in an animal model that closely resembles the human condition. ARC520 also has a straightforward and relatively short development path.
Valuation: New Value Ascribed To ARC-520
We have updated our valuation to include a contribution for ARC-520 for the first time, while revising assumptions for Arrowhead’s two other clinical stage assets. We now indicate a valuation for Arrowhead of $75m, of which ARC-520 accounts for 60%, reflecting its importance. The rNPV can be expected to rise significantly once proof-of-concept efficacy data on ARC-520 are generated in early 2014.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Arrowhead Research Corp.
Published 04/19/2013, 07:56 AM
Updated 07/09/2023, 06:31 AM
Arrowhead Research Corp.
ARC-520 Set To Become The Key Driver
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.