Allergy’s near-term investment case rests on M&A and successfully capitalising on potentially transformative regulatory catalysts. Confirmation of the lift of the FDA clinical hold on Pollinex Quattro (PQ) Grass, in place since 2007, is one of the latter; opening up the significant US allergy immunotherapy (AIT) opportunity. The FDA has also agreed the Phase III PQ Grass protocol, which means the development and regulatory pathway, costs and timelines are now better defined. This should facilitate Allergy’s efforts to secure a US development and commercialisation partner for PQ.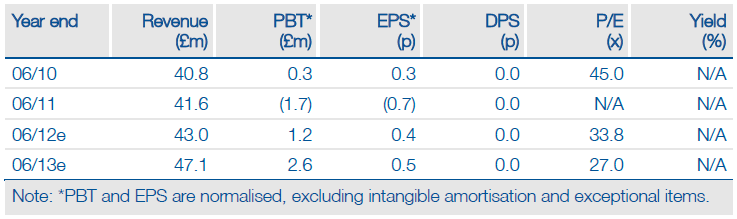
US regulatory overhang removed
FDA lift of the clinical hold and approval of a Phase III exposure chamber study for PQ Grass should enable Allergy to expedite the search for a US partner. The US AIT opportunity is significant (c $1.7bn), but is contingent on Allergy securing a partnership. This should boost the company’s growth post FY13. Currently, the US AIT market is 90% subcutaneous, but with no registered products; hence PQ should be well positioned to grow the market and overcome compliance, safety and reimbursement issues associated with allergen extracts.
European regulatory feedback expected next
German regulatory feedback on PQ Grass is pending, expected in H212; this could allow Allergy to proceed with its plans for a commercial launch in Germany in FY13. The ability to promote PQ Grass (currently only available on a named patient basis) has the potential to increase revenues. In addition, initial feedback on the 10 MAAs filed in 2011 should also start to come through from end-2012 onwards.
Valuation: US opportunity remains as upside
Management guidance at interims was for double-digit revenue growth in the next five years. There is improving visibility on the growth drivers, which have the potential to substantially boost revenues. The US is a significant additional opportunity; but it is not yet factored into the Edison revenue model. We intend to include this when a US partner is secured and there is visibility on both development timelines and deal economics. Allergy’s pro forma EV of c £61m (market cap adjusted for net debt of £7.4m at H112 and £13.5m in new funds raised in April) compares to an implied £52- 69m valuation range (DCF and peer analysis). The US remains pure upside at this stage.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Allergy Therapeutics Lift Of FDA clinical Hold
Published 08/22/2012, 02:20 AM
Updated 07/09/2023, 06:31 AM
Allergy Therapeutics Lift Of FDA clinical Hold
US opportunity beckons
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.