Recursion Pharmaceuticals Inc (NASDAQ:RXRX), the clinical-stage biotechnology company industrializing drug discovery by decoding biology, today reported business updates and financial results for its fourth quarter and fiscal year ending December 31, 2021.
"2021 was an exciting year for Recursion, in which we closed one of the largest biotechnology initial public offerings in history; expanded our partnership with Bayer (OTC:BAYRY); entered into a transformational partnership with Roche and Genentech; received Fast Track and Orphan Drug Designations from the FDA for our NF2 and FAP programs, respectively; readied several programs to initiate clinical trials; expanded our therapeutics pipeline with numerous programs in oncology; and built and began to utilize our supercomputer, BioHive1," said Recursion Co-Founder & CEO Chris Gibson, Ph.D. "As we applied our mapping and navigating capabilities to explore complex biology, we discovered and advanced many new scientific and business opportunities. We are excited for 2022 as we work to transition more biology and chemistry from maps to medicines."
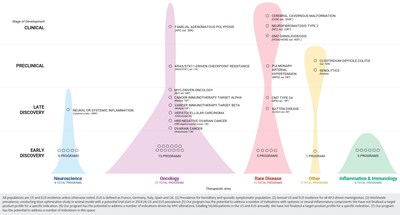
Summary of Business Highlights
- Clinical Programs
- Cerebral cavernous malformation (CCM) (REC-994): In March 2022, we enrolled the first patient in our Phase 2 SYCAMORE clinical trial, which is a double-blind, placebo-controlled safety, tolerability and exploratory efficacy study of this drug candidate in 60 subjects with CCM.
- Neurofibromatosis type 2 (NF2) (REC-2282): We plan to initiate our Phase 2/3 POPLAR-NF2 clinical trial, which is a parallel group, two stage, randomized, multicenter study of this drug candidate in the second quarter of 2022.
- Familial adenomatous polyposis (FAP) (REC-4881): We plan to initiate a Phase 2, randomized, double-blind, placebo-controlled study to evaluate safety, pharmacokinetics and efficacy of this drug candidate in the third quarter of 2022.
- Preclinical and Discovery (NASDAQ:DISCA) Programs
- Clostridium difficile colitis (REC-3964): We made progress in IND-enabling studies for REC-3964 and plan to initiate a Phase 1 study in the second half of 2022.
- Small molecule inhibitor of a target with a novel link to CDK12 biology: A small molecule inhibitor of a novel target not otherwise known to be related to CDK12, discovered using our next generation mapping and navigating technology, has demonstrated robust single-agent and combination activity with olaparib in an HRD-negative ovarian cancer PDX model, achieving 100% complete and durable response.
- Cancer immunotherapy target 'alpha': We expanded the in vivo dataset of target alpha, where a small molecule inhibitor of target alpha, discovered using our next generation mapping and navigating technology, demonstrated robust combination activity with an anti-PD1 therapy in an EMT6 mouse model and achieved 80% complete response.
- Oncology pipeline: We continued to make progress expanding and advancing numerous oncology programs, discovered using our next generation mapping and navigating technology, through scientific milestones including the programs mentioned above as well as programs related to immune checkpoint resistance in STK11-mutant non-small cell lung cancer, small molecule MYC inhibition, cancer immunotherapy target 'beta,' hepatocellular carcinoma, ovarian cancer, and other indications.
- Roche-Genentech Collaboration: In early December 2021, we announced a transformational collaboration with Roche and Genentech to advance novel potential medicines in neuroscience and an indication in gastrointestinal oncology by mapping complex biology using the Recursion OS. In this collaboration, Recursion received an upfront payment of $150 million in January 2022, is eligible for milestones for map-building and data-sharing that could exceed $500M, as well as research and development, commercialization and net sales milestones on up to 40 programs that could exceed $300M per program and mid- to high-single digit tiered royalties on net sales for products commercialized from this work together.
- Bayer AG (DE:BAYGN) Collaboration: In early December 2021, we announced the expansion of our collaboration with Bayer to include the use of Recursion's biological mapping and navigating capabilities to discover small molecule drug candidates with the potential to treat fibrotic diseases. In this expanded collaboration, Recursion and Bayer may now work on more than a dozen programs of relevance to fibrotic diseases.
- Recursion OS
- Closed Loop Automated Synthesis Suite (CLASS): We began designing CLASS, our automated chemical microsynthesis system, which will further enable novel chemical formulation and profiling across our maps of biology and chemistry.
- Total Observations: In the fourth quarter of 2021, we surpassed the milestone of executing 100 million total phenotypic experiments and producing 1 billion proprietary biological images.
Fourth Quarter and Fiscal Year 2021 Financial Results
- Cash Position: Cash, cash equivalents, and investments were $516.6 million as of December 31, 2021, compared to $262.1 million as of December 31, 2020. This cash balance does not include the upfront payment of $150 million from entering into the Roche-Genentech collaboration in December 2021, which was received in January 2022.
- Revenue: Total revenue, consisting primarily of revenue from collaborative agreements, was $2.5 million for the fourth quarter of 2021, compared to $2.7 million for the fourth quarter of 2020. Total revenue, consisting primarily of revenue from collaboration agreements, was $10.2 million for the year ended December 31, 2021, compared to $4.0 million for the year ended December 31, 2020. The increase in 2021 was due to revenue recognized from our collaboration with Bayer.
- Research and Development Expenses: Research and development expenses were $48.3 million for the fourth quarter of 2021, compared to $20.7 million for the fourth quarter of 2020. Research and development expenses were $135.3 million for the year ended December 31, 2021, compared to $63.3 million for the year ended December 31, 2020. The increases in both periods in 2021 were primarily due to an increased number of experiments run on the Recursion OS, an increased number of assets being validated, and increased clinical costs of studies to progress our drug candidates.
- General and Administrative Expenses: General and administrative expenses were $19.2 million for the fourth quarter of 2021, compared to $7.6 million for the fourth quarter of 2020. General and administrative expenses were $57.7 million for the year ended December 31, 2021, compared to $25.3 million for the year ended December 31, 2020. The increases in both periods in 2021 were due to the growth in size of the company's operations, including an increase in salaries and wages of $16.4 million during the year ended December 31, 2021, equipment costs, human resources-related costs, facilities costs, and other administrative costs associated with operating as a high-growth company.
- Net Loss: Net loss was $64.9 million for the fourth quarter of 2021, compared to a net loss of $25.8 million for the fourth quarter of 2020. Net loss was $186.5 million for the year ended December 31, 2021, compared to a net loss of $87.0 million for the year ended December 31, 2020.