Zynerba Pharmaceuticals, Inc. (NASDAQ:ZYNE) announced issuance of a new U.S. patent covering cannabidiol, a key compound of its lead pipeline candidate, Zygel, for treating autism spectrum disorder. The U.S. Patent and Trademark Office issued US Patent No. 10,314,792, which includes claims directed to methods of treating autism spectrum disorder through administration of a therapeutically effective amount of synthetic cannabidiol. The patent will expire in 2038.
Last month, the company initiated a phase II study to evaluate Zygel in pediatric patients with autism spectrum disorder.
We remind investors that Zynerba was awarded another methods of treating patent in February, covering synthetic or purified cannabidiol for the treatment of Fragile X syndrome, a genetic disorder causing certain developmental problems in patients. With the issuance of the new patent, the company owns 11 patents covering its pipeline products.
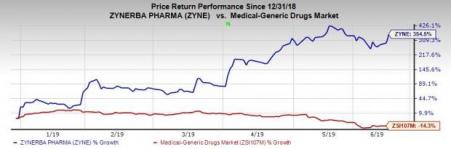
Shares of the company rallied 16.6% on Jun 11 as the new patent issuance strengthens its intellectual property portfolio covering Zygel. Zynerba’s shares have surged 354.5% so far this year against the industry’s decrease of 14.3%.
Please note that the company is evaluating Zygel for Fragile X syndrome, autism spectrum disorder in pediatric patients, 22q11.2 deletion syndrome, and developmental and epileptic encephalopathies in multiple clinical studies in different stages of development. With the issuance of two patents this year, the candidate will be shielded from competition till 2038 upon successful development in Fragile X syndrome and autism spectrum disorder indications.
The legalization of medical marijuana in multiple states in the United States suggests that the availability of cannabis-based products like cannabidiol is likely to increase. Several companies focused on developing similar products will likely have sufficient supply of cannabis, which will likely make the segment competitive. Patents will help Zynerba to offset competition for its products in approved indications.
Zynerba initiated a pivotal phase II/III study evaluating Zygel in pediatric patients with Fragile X syndrome last year. Top-line results from the study are expected in the second half of 2019 with potential new drug application submission in the first half of 2020. Previously presented data from an open-label phase II study showed improvement in core emotional and behavioral symptoms of statistical significance in patients with Fragile X syndrome. In May, the FDA granted Fast Track designation to the Zygel for this indication.
A phase II study is evaluating the candidate in developmental and epileptic encephalopathies. Top-line data is expected in the third quarter. In addition to autism spectrum disorder, the company initiated another phase II study last month to evaluate Zygel as a treatment for 22q11.2 deletion syndrome, a genetic disorder which may lead to mental illness.
We note that a few companies have cannabidiol drugs in their portfolio. The market is expected to reach $22 billion by 2022 per a report. GW Pharmaceuticals’ (NASDAQ:GWPH) cannabidiol drug Epidiolex was the first medicine made from Cannabis sativa plant species to win approval from the FDA. The drug is primarily used to treat rare forms of epilepsy. Insys Therapeutics (NASDAQ:INSY) is developing a cannabidiol oral solution for epilepsy, infantile spasms and Prader-Willi syndrome. However, the company filed for bankruptcy earlier this week.
Zacks Rank & Stock to Consider
Zynerba currently carries Zacks Rank #3 (Hold). Anika Therapeutics Inc. (NASDAQ:ANIK) is a better- ranked biotech stock, sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Anika’s earnings per share estimates have increased from $1.22 to $1.28 for 2019 and from $1.21 to $1.33 for 2020 over the past 60 days. The stock has rallied 18.2% so far this year.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Insys Therapeutics, Inc. (INSY): Free Stock Analysis Report
GW Pharmaceuticals PLC (GWPH): Free Stock Analysis Report
Anika Therapeutics Inc. (ANIK): Free Stock Analysis Report
Zynerba Pharmaceuticals, Inc. (ZYNE): Free Stock Analysis Report
Original post