Zealand Pharma A/S (NASDAQ:ZEAL) announced that it has initiated the second phase III study to evaluate its pipeline candidate, dasiglucagon, in pediatric patients with congenital hyperinsulinism (“CHI”).
The CHI disease is a rare pediatric disease, primarily affecting newborns, infants and toddlers, which can lead to debilitating lifelong complications like high insulin levels. Treatment options currently available are often insufficient and necessitate surgical intervention through pancreatectomy.
Shares of Zealand were up 7.1% on Dec 4 following the announcement. The company’s shares have surged 181.7% so far this year compared with the industry’s increase of 5.3%.
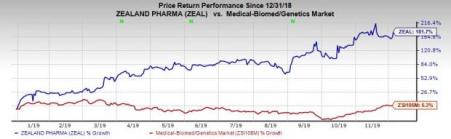
The new phase III study is designed to evaluate dasiglucagon in 12 CHI patients who are aged between 7 days to 1 year and are dependent upon intravenous glucose infusions to maintain their plasma glucose levels. The efficacy of the candidate will be measured on the basis of reduction in the intravenous glucose infusion rate, number of hypoglycemic events, and total amount of carbohydrate administered.
An ongoing phase III study is evaluating dasiglucagon versus current standard treatments for reducing the number of hypoglycemic events in CHI patients aged 3 months to 12 years. Results from this study are expected next year.
We note that dasiglucagon enjoys orphan drug designation both in the United States and Europe for treating CHI patients. The FDA also granted a rare pediatric disease designation in 2019 for this indication.
Apart from CHI, Zealand is also developing dasiglucagon across several indications including severe hypoglycemia and diabetes management.
In September, the company announced positive results from the pediatric phase III study evaluating dasiglucagon for severe hypoglycemia in diabetes. Dasiglucagon is a potential first-in-class soluble glucagon analog, which is being developed in the ready-to-use HypoPal rescue pen. Data from the study demonstrated that patients treated with the candidate improved the time to increase in plasma glucose of greater than equal to 20 mg/dL (1.1 mmol/L) from baseline without administration of rescue intravenous glucose.
Zacks Rank & Stocks to Consider
Zealand currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks to consider from the biotech sector are Alkermes plc (NASDAQ:ALKS) , Anika Therapeutics Inc. (NASDAQ:ANIK) and BioDelivery Sciences International, Inc. (NASDAQ:BDSI) . While Alkermes and Anika sport a Zacks Rank #1 (Strong Buy), BioDelivery carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alkermes’ earnings estimates increased from 36 cents to 52 cents for 2019 and changed from a loss of 11 cents to earnings of 59 cents for 2020 over the past 60 days. The company delivered a positive earnings surprise in the trailing four quarters, the average beat being 236.8%.
BioDelivery’s loss estimates have narrowed from 26 cents to 16 cents for 2019. For 2020, earnings estimates moved up from 19 cents to 31 cents for 2020 over the past 60 days. The company delivered a positive earnings surprise in three of the trailing four quarters, the average beat being 159.38%. Share price of the company has increased 81.1% so far this year.
Anika’s earnings estimates increased from $1.75 to $2.03 for 2019 and from $1.38 to $1.62 for 2020 over the past 60 days. The company delivered a positive earnings surprise in all the trailing four quarters, the average beat being 53.31%. Share price of the company has increased 69.8% so far this year.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Anika Therapeutics Inc. (ANIK): Free Stock Analysis Report
Alkermes plc (ALKS): Free Stock Analysis Report
BioDelivery Sciences International, Inc. (BDSI): Free Stock Analysis Report
Zealand Pharma A/S (ZEAL): Free Stock Analysis Report
Original post
Zacks Investment Research