Völlig baff (gobsmacked) survival
Redectane and Mesupron are now Wilex’s (WL6.DE) key products after the Rencarex Phase III ARISER data showed no efficacy; Rencarex has been discontinued. After the July ODAC meeting determined that a hypothetical imaging test for clear cell renal carcinoma would be clinically useful, the FDA agreed that a Redectane confirmatory study could lead to a US filing. The design and timing of the REDECT-2 Phase III are being finalised. Mesupron has good Phase II data in both pancreatic and HER-2 negative breast cancer and could be partnered in 2013. Wilex is funded into 2014; Q3 cash was €28.7m.
Principle not product
The Oncology Drugs Advisory Committee (ODAC) recommended that a hypothetical imaging agent to identify clear-cell renal cell carcinoma (ccRCC) would “speak for itself” and be clinically relevant. The Redectane REDECT Phase III study (reported in 2010), showed sensitivity of 86% (correct diagnosis of ccRCC, p≤0.016) with a specificity of 87% (correct exclusion of ccRCC; p
Rencarex results
The ARISER trial enrolled 864 patients, mostly at T3 or T4 stage (where the tumour has grown out of the kidney). These patients are at high risk of relapse so the trial was expected to end within a few years. The low event rate created the possibility that the therapy reduced metastasis but Rencarex showed no efficacy. The low event rate may have been due to careful exclusion of most metastatic patients from the study: many patients may have been cured by surgery so Rencarex could have no effect.
Q3 financial position
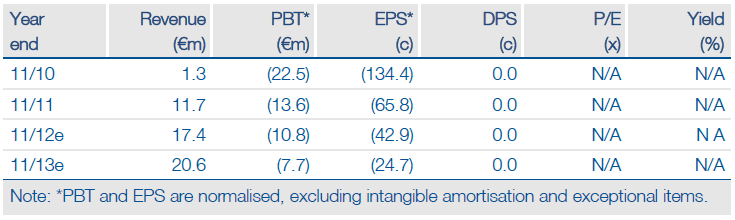
Wilex’s annual cash need after sales margins has been indicated at €20-24m. Q3 revenues to 31 August were €11.3m with other income of €1.5m: €12.8m total. €9.7m of the revenues arose from part recognition of Prometheus’s Rencarex payments with €1.6m in sales from the Heidelberg and diagnostics operations.
Valuation: Rests on Mesupron deal in 2013
The indicative value of Wilex is under review after the Rencarex failure, but a provisional value is likely to be in the €1.20-1.50/share range, materially reduced. The value of some technologies (like ADC) is not included but could be significant. A Mesupron deal is expected in 2013 after good trend data were seen in both the pancreatic and breast cancer studies. This could boost Wilex’s value to perhaps the €2.00-2.50/share range.
To Read the Entire Report Please Click on the pdf File Below.
Redectane and Mesupron are now Wilex’s (WL6.DE) key products after the Rencarex Phase III ARISER data showed no efficacy; Rencarex has been discontinued. After the July ODAC meeting determined that a hypothetical imaging test for clear cell renal carcinoma would be clinically useful, the FDA agreed that a Redectane confirmatory study could lead to a US filing. The design and timing of the REDECT-2 Phase III are being finalised. Mesupron has good Phase II data in both pancreatic and HER-2 negative breast cancer and could be partnered in 2013. Wilex is funded into 2014; Q3 cash was €28.7m.

Principle not product
The Oncology Drugs Advisory Committee (ODAC) recommended that a hypothetical imaging agent to identify clear-cell renal cell carcinoma (ccRCC) would “speak for itself” and be clinically relevant. The Redectane REDECT Phase III study (reported in 2010), showed sensitivity of 86% (correct diagnosis of ccRCC, p≤0.016) with a specificity of 87% (correct exclusion of ccRCC; p
Rencarex results
The ARISER trial enrolled 864 patients, mostly at T3 or T4 stage (where the tumour has grown out of the kidney). These patients are at high risk of relapse so the trial was expected to end within a few years. The low event rate created the possibility that the therapy reduced metastasis but Rencarex showed no efficacy. The low event rate may have been due to careful exclusion of most metastatic patients from the study: many patients may have been cured by surgery so Rencarex could have no effect.
Q3 financial position
Wilex’s annual cash need after sales margins has been indicated at €20-24m. Q3 revenues to 31 August were €11.3m with other income of €1.5m: €12.8m total. €9.7m of the revenues arose from part recognition of Prometheus’s Rencarex payments with €1.6m in sales from the Heidelberg and diagnostics operations.
Valuation: Rests on Mesupron deal in 2013
The indicative value of Wilex is under review after the Rencarex failure, but a provisional value is likely to be in the €1.20-1.50/share range, materially reduced. The value of some technologies (like ADC) is not included but could be significant. A Mesupron deal is expected in 2013 after good trend data were seen in both the pancreatic and breast cancer studies. This could boost Wilex’s value to perhaps the €2.00-2.50/share range.
To Read the Entire Report Please Click on the pdf File Below.