I spend a fair bit of time looking at smaller biotech companies that are working towards providing better treatments for existing diseases.
These companies offer a lot of potential but they also carry a very high level of risk. It is as they say the nature of the beast.
I suppose this is why I find VBI Vaccines Inc (NASDAQ:VBIV) so intriguing. The company has the same potential that this sector typically offers but it comes with a lot less risk. I’ll explain why in a moment.
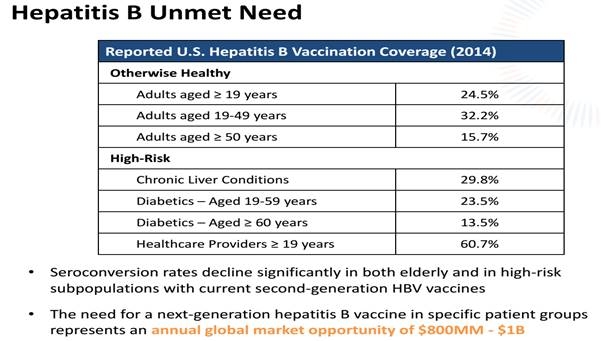
VBI is looking to provide a solution for a specific group of patients that are currently not suitable for Hepatitis B vaccination. A $1 billion opportunity.
Source of image: VBI Vaccines Presentation
Hepatitis-B is nasty business. The key facts about Hepatitis-B according to the World Health Organization are as follows:
- Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic disease.
- The virus is transmitted through contact with the blood or other body fluids of an infected person.
- An estimated 240 million people are chronically infected with hepatitis B (defined as hepatitis B surface antigen positive for at least 6 months).
- More than 686 000 people die every year due to complications of hepatitis B, including cirrhosis and liver cancer 1.
- Hepatitis B is an important occupational hazard for health workers.
- Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus. It is a major global health problem. It can cause chronic infection and puts people at high risk of death from cirrhosis and liver cancer.
In North American and Europe GlaxoSmithKline's (NYSE:GSK)(GSK) Engerix-B is the current standard of care for Hepatitis B vaccines. Engerix-B works fine for young, healthy people.
It is not however suitable for people with liver conditions, diabetics and people over 40 with weaker immune systems.
For these people VBI Vaccines has a solution. I’m not talking about a vaccine in development, I’m talking about a vaccine (Sci-B-Vac) that has already been proven to be effective.
This is the lower level of risk that VBI presents which I mentioned earlier.
This point was brought forward once again with VBI’s press release concerning its Phase IV study on 88 seronegative people (without previous immunity to Hepatitis B). Of the 88 subjects in the study data revealed that 98.8% of participants were seroprotected at Month 3, two months after receiving a second dose of Sci-B-Vac, and prior to receiving a third dose.
These results should not come as a surprise. Sci-B-Vac has already been proven through more than 20 clinical trials and has been used by more than 4,500 patients. The vaccine is the standard of care in Israel and has been approved for use in 15 countries.
Therein lies my interest in VBI. A company that has already developed a solution and just needs to bring it to market. A much lower risk business proposition than a typical biotech which is offers potential but more uncertainty.
Bringing this vaccine to market is exactly why VBI Vaccines and SciVac Therapeutics merged earlier this year. SciVac had the vaccine and VBI provided the financial and corporate capability to bring that vaccine to the larger markets in the United States and Europe.
Other Products In The Pipeline
Sci-B-Vac is not VBI’s only product under development. There are also a couple of others which offer potential but more risk.
#1 - CMV (Cytomegalovirus) Vaccine
CMV is a very common virus that impacts one in every two people in many parts of the developing world. Most infections of CMV are "silent" which means that there are no symptoms or signs. You have it but you don't know.
VBI is developing a prophylactic vaccine to prevent cytomegalovirus (CMV) infection.
CMV isn't really a problem for most of us. Where it becomes a problem is when a mother is infected during pregnancy because it can result in serious consequences for the newborn. When this happens it is known as a congenital CMV infection.
5,000 U.S. infants will develop permanent problems due to CMV every year on average. Some of these problems are quite severe, including deafness, blindness, and mental retardation.
CMV impacts more births than either Down Syndrome or Fetal Alcohol Syndrome. CMV is a key public health priority that needs a solution and would be a strong candidate for recommended universal vaccination among certain high-risk populations.
There is no vaccine for prevention of CMV. VBI is closest in the race to develop one since it is a year to 18 months ahead of its nearest competitor vaccine which is being developed by Merck (NYSE:MRK)
Should VBI successfully develop a fully tested and approved vaccine there is likely to be widespread public health support for it.
The market for this product is estimated to be $1.5 billion in the United States (based on number of births and $100 per vaccination) with a similar sized opportunity in developed markets outside the United States.
That would give VBI the head start on a $3 billion market.
#2 - Glioblastoma Immunotherapy
Glioblastoma is one of the most common and aggressive malignant primary brain tumors that a human can suffer from. In the United States alone, 12,000 new cases of Glioblastoma are diagnosed each year.
The current standard of care for GBM is a surgery, followed by radiation and chemotherapy. Unfortunately even with this aggressive treatment, GBM progresses rapidly and is exceptionally lethal. The median patient survival is less than sixteen months.
VBI has applied its eVLP Platform to the development of a glioblastoma therapeutic vaccine candidate. With its unique approach, VBI is trying to create a GBM immunotherapy that will stimulate the patient's own immune system to identify and kill GBM cancer cells. The ultimate goal is to create a commercially-viable therapy that is more effective and tolerable than current treatments.
Risks – While I definitely like the reduced amount of risk involved with the Sci-B-Vac vaccine this opportunity is not risk free. The nature of this industry is one where competition is fierce and changing quickly. Please keep that in mind when investing in these types of companies.