Theravance Biopharma, Inc. (NASDAQ:TBPH) announced promising outcomes in the areas of safety and tolerability, pharmacokinetics and preliminary pharmacodynamic activity from a phase I study on its investigational, inhaled, lung-selective pan-Janus kinase (JAK) inhibitor, TD-8236. The candidate is being developed for the treatment of inflammatory lung diseases with minimal systemic exposure.
The early-stage randomized, placebo-controlled study on TD-8236 consists of two parts. The first evaluated the candidate in single ascending doses (SAD) (up to 4500 mcg) in healthy patients while the second evaluated TD-8236 in multiple ascending doses (MAD) (up to 4000 mcg), which is administered as a once-daily dose to patients with mild asthma for seven consecutive days.
Initial data from the study showed that in both regimens, TD-8236 was well tolerated with no evidence of local irritation or bronchoconstriction in the given patient population. Also, none of the patients discontinued the treatment and there were no serious adverse side effects reported.
Importantly, the candidate produced the desired biological activity in the target patient population as it led to reduction in fractional exhaled nitric oxide (FeNO) in mild asthma patients who had elevated levels of FeNO. Treatment with TD-8236 also resulted in minimal systemic exposure.
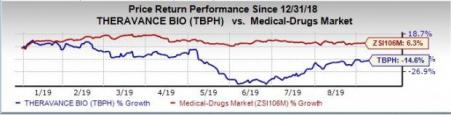
Shares of Theravance were up almost 1.4% post this news on Monday. However, the stock has declined 14.6% so far this year against the industry’s increase of 6.3%.
TD-8236 is particularly designed as a dry-powder inhaler for the lungs with minimal systemic exposure. Following positive results from this study, Theravance has initiated a Part C extension portion of the phase I study on TD-8236, which will assess a range of additional biomarkers in patients with more severe asthma. The company also plans to initiate a lung allergen challenge phase II study on the candidate to provide additional insights in the days ahead.
We would like to remind investors that last November, Theravance dosed the first patient in the phase I study on TD-8236. It is the second JAK inhibitor discovered by Theravance.
Another JAK inhibitor in Theravance’s portfolio is TD-1473, which is being developed for the treatment of inflammatory intestinal diseases. The candidate is currently being evaluated in a phase IIb/III study for treating moderate-to-severe ulcerative colitis and in a phase II study for Crohn’s disease. The company has a collaboration agreement with Johnson & Johnson’s (NYSE:JNJ) subsidiary Janssen for developing TD-1473.
Theravance plans to report data from the phase IIb portion of the ulcerative colitis and phase II Crohn's disease studies on TD-1473 by late 2020.
Zacks Rank & Other Stocks to Consider
Theravance currently carries a Zacks Rank #2 (Buy). Other top-ranked stocks in the same sector include Cumberland Pharmaceuticals Inc. (NASDAQ:CPIX) and FibroGen, Inc (NASDAQ:FGEN) , both sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Cumberland Pharmaceuticals’ earnings estimates have moved 8.1% north for 2019 and 6.5% for 2020 over the past 60 days.
FibroGen’s loss per share estimates have been narrowed 57.5% for 2019 and 64.2% for 2020 over the past 60 days.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Johnson & Johnson (JNJ): Free Stock Analysis Report
FibroGen, Inc (FGEN): Free Stock Analysis Report
Cumberland Pharmaceuticals Inc. (CPIX): Free Stock Analysis Report
Theravance Biopharma, Inc. (TBPH): Free Stock Analysis Report
Original post
Zacks Investment Research
