In January 2019, Sunesis Pharmaceuticals (NASDAQ:SNSS) announced that its Phase Ib/II study of vecabrutinib for B-cell cancers advanced to the 100mg cohort. The company experienced a series of unavoidable clinical delays in the 50mg arm but was eventually able expand the number of clinical sites and overenroll the cohort. The 100mg dose is the first that is expected to potentially provide indications of efficacy and Sunesis will provide a clinical update in Q219.
Advancement In Line With Expectations
The announcement of the advancement of the Phase Ib/II study confirms both company guidance and our expectations. The delays in the 50mg arm of the study were not indicative of any issues with the drug in our assessment and instead were a reflection of unavoidable clinical risks. These included a per-protocol cohort expansion followed by early progression in a series of patents. As the 50mg dose was below the effective dose (expected between 100mg and 300mg), it was too early to draw conclusions on safety or efficacy, but the upcoming 100mg dose should provide more insight.
Further Progress Expected To Be Smoother
The company instituted a series of amendments to its clinical trial in 2018 that should enhance the ability of the program to progress smoothly. It added three clinical sites, bringing the total to eight. This should aid enrolment, and the company was able to overenroll the 50mg cohort. We expect the company to continue to overenroll. Additionally, Sunesis expanded the indications being examined in the study to include diffuse large B-cell lymphoma and follicular lymphoma. Although no patients from these indications yet have been treated, we expect inclusion to also improve enrolment and for any data on these diseases to illuminate to potential future indications beyond the main target of chronic lymphocytic leukemia (CLL).
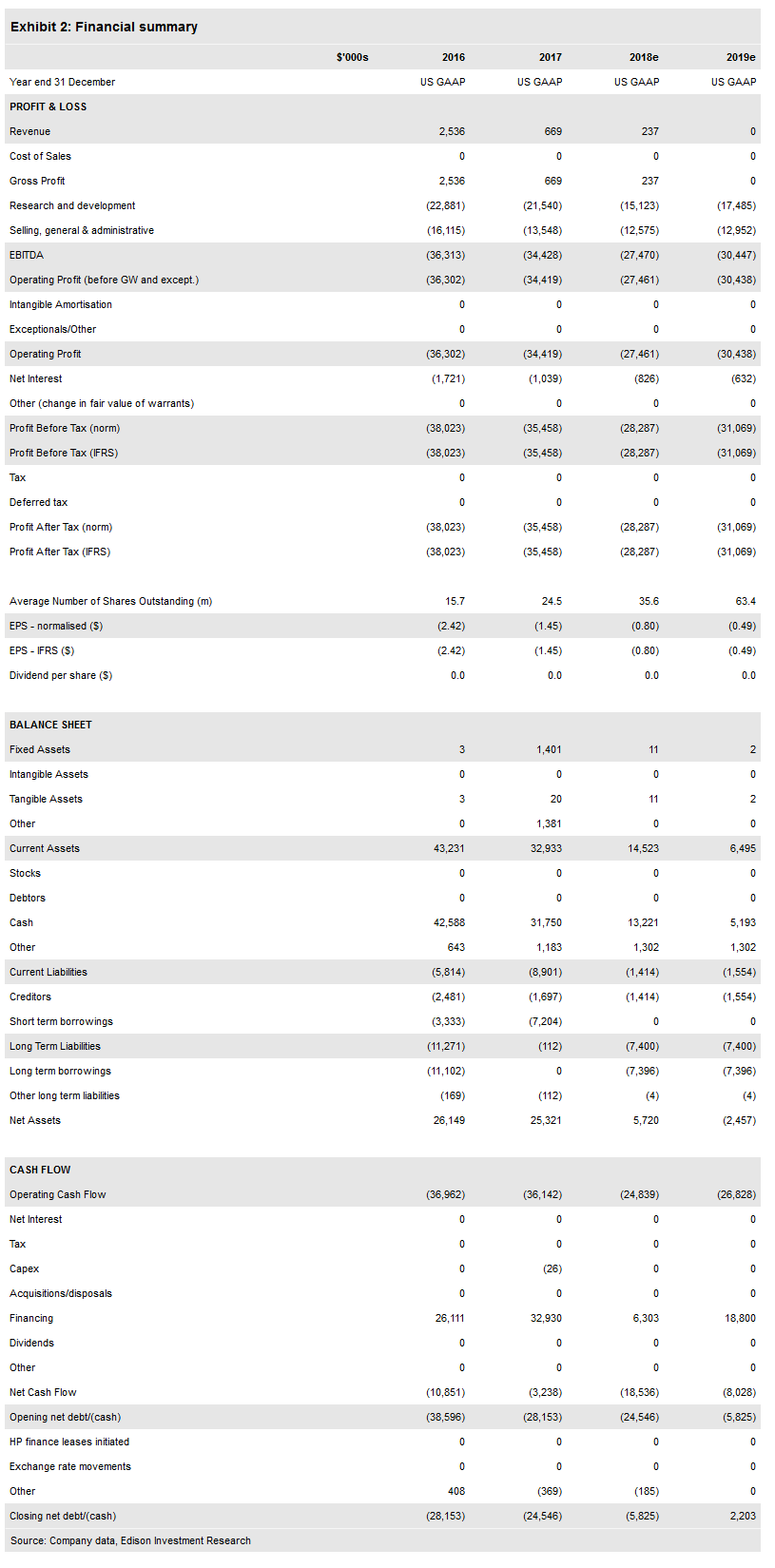
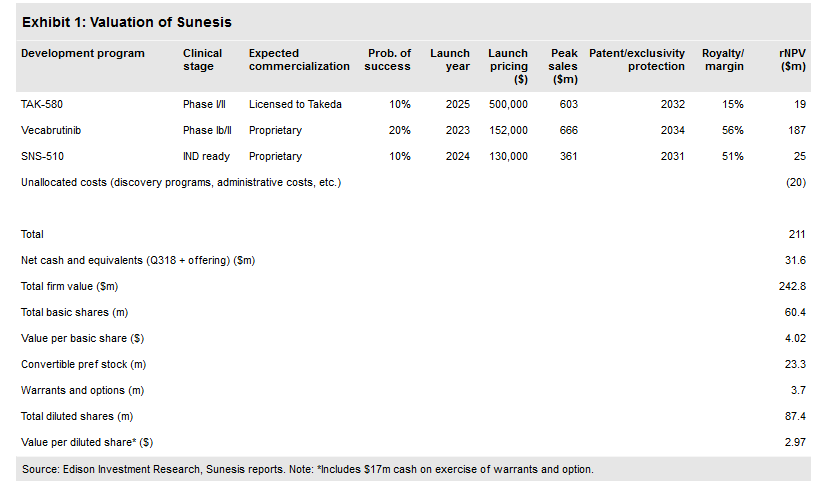
Valuation: $243m or $4.02 ($2.97 diluted)
Following the recent financing, our valuation is now $243m or $4.02 per basic share ($2.97 diluted) from $224m and $5.99 ($4.98 diluted). The company priced an offering of 23m shares of common stock and 17,000 shares of convertible preferred stock (equivalent to 17m common) at $0.50 per common share or equivalent for gross proceeds of $20m, providing a cash runway into 2020. We expect the company to require an additional $115m before profitability in 2023.
Business Description
Sunesis Pharmaceuticals is a pharmaceutical company focused on oncology. Its lead asset is vecabrutinib, a Bruton’s tyrosine kinase inhibitor for chronic lymphocytic leukemia for Imbruvica-refractory patients. The program is entering a dose escalation Phase Ib/II. It has also developed TAK-580 with partner Takeda, and the preclinical PDK1 inhibitor SNS-510.
100mg At Long Last
In early January 2019 Sunesis announced that the ongoing Phase Ib/II study of vecabrutinib had advanced to the 100mg dosing cohort. The company quickly filled the three required slots in early January, with the first doses expected to be received in the following two to four weeks. The BTK inhibitor is being examined for CLL and a series of other B-cell malignancies, and the previous 50mg cohort was plagued by unforeseen and unavoidable delays throughout 2018. An ALT elevation prevented a patient from receiving the required number of doses, triggering a cohort expansion to six patients (per the 3+3 dose escalation protocol). Of the new patients, three progressed before they could be evaluated. In 2018 the company expanded the number of clinical sites on the study (to eight), which should improve enrolment; this may have been reflected in the fact that the company was able to ultimately overenroll the 50mg cohort. If the company continues to overenroll the 100mg and later arms, this should hedge against further setbacks. The increase in clinical sites is also important for the eventual progression of the trial to the Phase II portion of the study, once the effective dose has been identified.
The company expects the active dose of the drug to be found in the range of 100mg to 300mg, meaning the 100mg cohort could show signs of efficacy. Even if 100mg is not the optimal dose, we expect increasing signs of clinical activity; at the 50mg level, the company presented data showing a reduction in cytokine production (ASH 2018), a downstream indicator of BTK inhibition. Given that the clinical mechanism of BTK inhibition has already been vetted with Imbruvica (ibrutinib), we find even early signs of activity to be highly encouraging. The company stated that it will provide a clinical update at a medical conference in Q219.
Valuation
Our valuation has increased to $243m from $224m following the stock offering in January, but has decreased on a per share basis to $4.02 per basic share ($2.97 diluted) from $5.99 ($4.98 diluted). Otherwise our model remains unchanged. We expect to update our valuation with increasing evidence regarding the activity of vecabrutinib, and advancement to the Phase II portion of the study.
Financials
On 17 January, the company announced the pricing of the offering of common and preferred stock with gross proceeds of $20m. The offering included 23m shares of common stock and 17,000 shares (equivalent to 17m common shares) of convertible preferred stock at an offering price of $0.50 per common share or equivalent. Based on our financial projections, this should provide a cash runway throughout 2019 and into 2020. The company ended Q318 with $20.2m in cash (and $7.3m in debt). We have adjusted our financing schedule as a result and expect the company to need $115m in additional capital to reach profitability in 2023 ($40m in 2020, $40m in 2021, $35m in 2022), down from $135m previously.