Rigel Pharmaceuticals, Inc. (NASDAQ:RIGL) has enrolled the first patient in a pivotal phase III study evaluating Tavalisse (fostamatinib disodium hexahydrate) as a treatment for warm antibody autoimmune hemolytic anemia (“AIHA”). The company expects to complete enrollment in a year.
The study will evaluate the oral spleen tyrosine kinase (“SYK”) inhibitor, Tavalisse, in approximately 80 patients with primary or secondary warm AIHA, who have failed at least one prior treatment, over a period of 24 weeks. Top-line data from the pivotal study is expected in 2021, which will likely form the basis of regulatory application for label expansion of the drug to include AIHA patients.
One of the two primary endpoints of the study is to achieve a durable hemoglobin response measured by hemoglobin levels of more than 10 g/dL and an improvement of 2 g/dL from baseline. Another primary endpoint of the study is the durability of response measure.
The company stated that the rare, serious blood disorder AIHA affects approximately 40,000 patients in the United States. The disorder can become a severe, debilitating disease wherein the patients’ immune system produces antibodies that destroy the body's red blood cells. There are no FDA-approved therapies targeting the disease.
Rigel has completed the phase II study – SOAR – evaluating Tavalisse in similar patient population with AIHA for identical primary endpoints as the phase III study. Data from the study showed that 43% of patients achieved hemoglobin levels of greater than 10 g/dL and at least a 2 g/dl increase from baseline. An open-label extension of the SOAR study is ongoing.
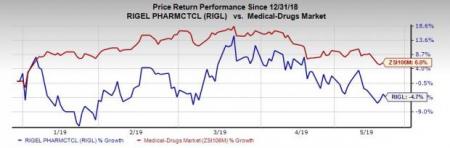
Rigel’s stock has declined 4.7% in the year so far against the industry’s rise of 6%.
We remind investors that Tavalisse was approved in April 2018 in the United States for the treatment of chronic immune thrombocytopenia in adult patients with inadequate response to the previous treatment. The drug is the only marketed drug in the company’s portfolio. The drug has generated more than $20 million in sales in the first 10 months since its launch. The drug is under review in Europe for chronic immune thrombocytopenia. A decision is expected by the end of 2019.
Rigel also has an early-stage pipeline candidate – R835, an interleukin receptor associated kinase inhibitor – which is being developed for autoimmune and inflammatory diseases. The company also has several partnered programs in collaboration with companies like AstraZeneca (NYSE:AZN) and Aclaris Therapeutics (NASDAQ:ACRS) in early to mid-stage studies targeting inflammatory and cancer indications.
In the same press release, the company announced that Anne-Marie Duliege will be resigning from the posts of executive vice president and chief medical officer, effective Aug 31, 2019. The company is looking for a replacement for Duliege.
Zacks Rank & Key Pick
Rigel currently carries a Zacks Rank #2 (Buy). KalVista Pharmaceuticals, Inc. (NASDAQ:KALV) is another stock to consider from the drug industry, carrying a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
KalVista’s loss estimates have narrowed 8.3% for 2019 and 6.3% for 2020 over the past 60 days. The stock has gained 23.4% so far this year.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to iterally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98%, +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
AstraZeneca PLC (AZN): Free Stock Analysis Report
KalVista Pharmaceuticals, Inc. (KALV): Free Stock Analysis Report
Rigel Pharmaceuticals, Inc. (RIGL): Free Stock Analysis Report
Aclaris Therapeutics, Inc. (ACRS): Free Stock Analysis Report
Original post
Zacks Investment Research