ReNeuron Group PLC (LON:RQE)’s announcement of early but exciting data on three patients in the clinical study of its human retinal progenitor cell (hRPC) product was well received. Although the patient numbers are small and the follow-up time points are short (two months and 18 days), the improvements in visual acuity are striking and may enable an earlier start to the pivotal programme.
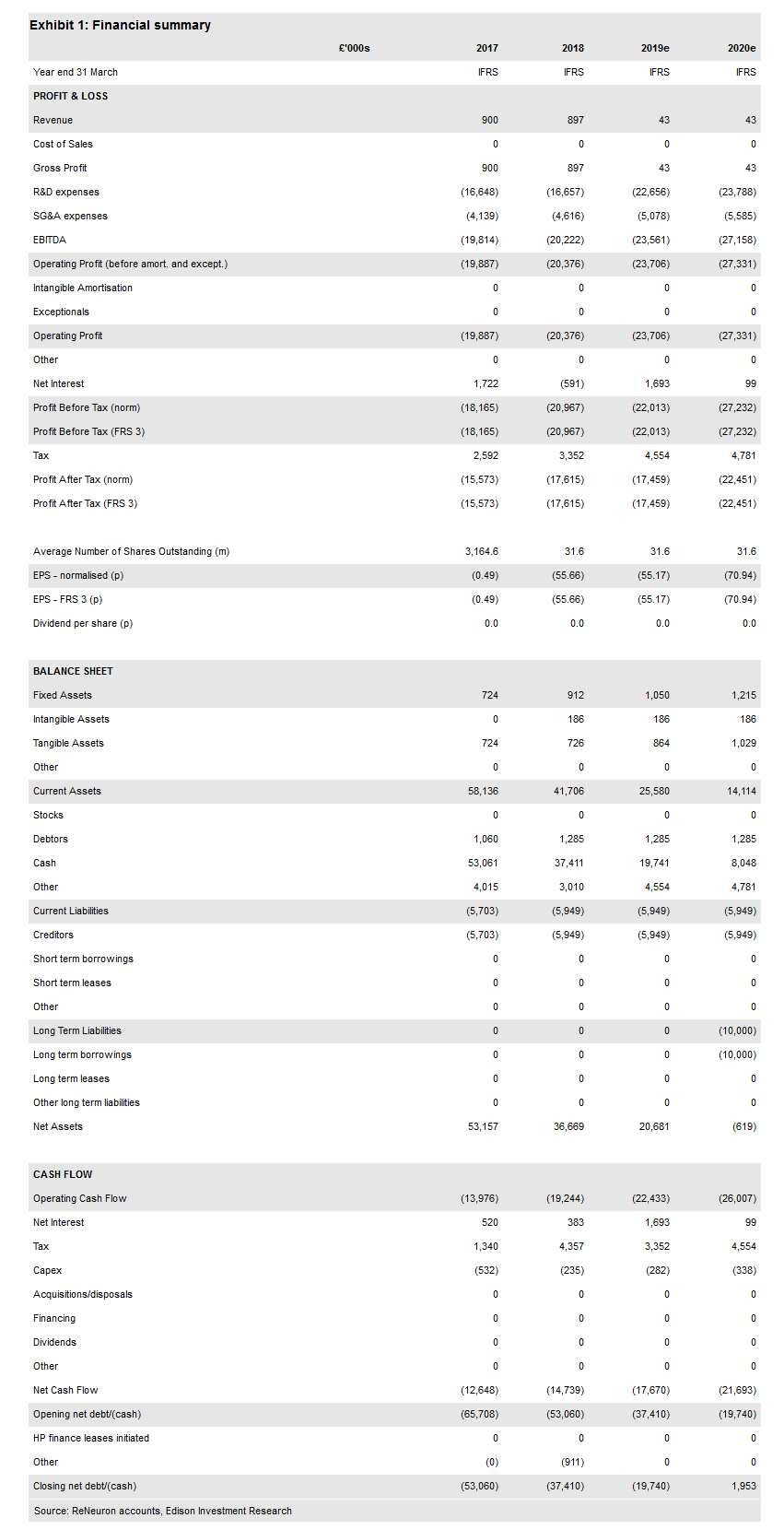
Positive preliminary data in RP
ReNeuron has announced positive data on the first three RP patients in the Phase I/IIa clinical study of its hRPC cell therapy product. At this early stage in the study our expectations were limited as we had anticipated that more time would be required for efficacy to be seen. A surprising and dramatic improvement in visual acuity was observed – a 20-letter improvement at two months, 15- and 14-letter improvements at 18 days – in the first three patients forming the first cohort dosed with the new cryopreserved, commercially ready formulation. Investors will be familiar with the letters on an ETDRS eye chart from their regular eye tests. As a comparison, albeit in different indications, the best-selling drug, Eylea, for wet age-related macular degeneration (wAMD) and diabetic macular oedema (DME), has only shown maintenance (rather than improvement) of visual acuity in 94% of wAMD patients and a 9.6- to 18.8-letter gain in DME depending on the severity of disease. These data from ReNeuron’s study are measured from baseline but also use the patient’s untreated eye as a placebo.
What came before and what comes after?
In the first Phase I portion of the study, ReNeuron had already dosed 12 patients with more severe disease (lower baseline acuity) with an earlier formulation that was not optimised for shelf life or subretinal implantation. This gave regulators a safety baseline to allow study progression to less severe patients (where visual acuity improvements should be more obvious) and higher doses. With another three patients expected to start in March, again at the top dose from the Phase I study portion, if efficacy is also demonstrated in this cohort and acuity gains in the first cohort are maintained, ReNeuron will be in a good position to approach regulators and potential partners to discuss an accelerated path to market.
Valuation: No changes for now
We have made no changes to our valuation or probabilities at this stage. However, we recognise that early evidence of efficacy may enable ReNeuron to advance to a pivotal study more quickly, subject to discussions with regulators. This is because, for many RP patients, no therapeutic options exist. Our forecasts and valuation are unchanged at £192m or 608p per share.
Business description
ReNeuron is a UK biotech company developing allogeneic cell therapies. The first pivotal Phase IIb trial for CTX neural stem cells for chronic stroke disability is underway. Human retinal progenitor cells (hRPCs) are also being studied for RP (in Phase I/IIa).