Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) and Sanofi’s (NASDAQ:SNY) supplemental Biologics License Application (sBLA) for asthma drug Dupixent (dupilumab) has been accepted by the FDA for Priority Review. The company is seeking approval of Dupixent as an add-on maintenance treatment for children 6-11 years with moderate-to-severe atopic dermatitis (AD), whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The FDA set an action date of May 26, 2020. The FDA previously granted Breakthrough Therapy designation to Dupixent for the same indication.
The sBLa is supported by the phase III results on the efficacy and safety of the drug combined with topical corticosteroids (TCS) in children with severe atopic dermatitis. The data showed that children treated with Dupixent and TCS experienced significantly improved overall disease severity, skin clearing, itching and health-related quality of life compared with TCS alone.
We remind investors that Dupixent is approved in the United States to treat patients aged 12 years and older with moderate-to-severe AD that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. It is also approved for use with other medicines to treat chronic rhinosinusitis with nasal polyposis (CRSwNP) in adults, whose disease is not controlled. It is also approved for use with other asthma medicines for the maintenance treatment of moderate-to-severe eosinophilic or oral steroid-dependent asthma in patients aged 12 years or above, whose asthma is not controlled with their current medicines.
Outside the United States, the drug is approved for specific patients with moderate-to-severe atopic dermatitis and certain patients with asthma in the EU and Japan. Dupixent is also approved in the EU to treat certain adults with severe CRSwNP.
Label expansion of the drug will diversify the company’s revenue base and reduce dependence on lead drug, Eylea.
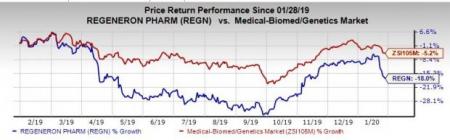
Regeneron’s stock has lost 18% in the past year compared with the industry’s decline of 5.2%.
The company’s efforts to develop its pipeline and diversify the revenue base are impressive. It earlier extended the collaboration agreement with Alnylam Pharmaceuticals, Inc. (NASDAQ:ALNY) to discover, develop and commercialize new RNA interference (RNAi) therapeutics for a broad range of diseases by addressing disease targets expressed in the eye and central nervous system (CNS), in addition to a select number of targets expressed in the liver.
Zacks Rank & A Stock to Consider
Regeneron currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the same space is ASLAN Pharmaceuticals Ltd. (NASDAQ:ASLN) , which carries a Zacks Rank #1 (Strong Buy), currently. You can see the complete list of today’s Zacks #1 Rank stocks here.
ASLAN’s loss per share estimates have narrowed from 69 cents to 67 cents for 2019 and from 62 cents to 61 cents for 2020 over the past 60 days. The company delivered a positive earnings surprise of 24.65%, on average, in three of the last four quarters.
5 Stocks Set to Double
Each was hand-picked by a Zacks expert as the #1 favorite stock to gain +100% or more in 2020. Each comes from a different sector and has unique qualities and catalysts that could fuel exceptional growth. Most of the stocks in this report are flying under Wall Street radar, which provides a great opportunity to get in on the ground floor.
Today, See These 5 Potential Home Runs >>
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Alnylam Pharmaceuticals, Inc. (ALNY): Free Stock Analysis Report
ASLAN Pharmaceuticals Ltd. (ASLN): Free Stock Analysis Report
Original post
Zacks Investment Research