Quantum Genomics (PA:ALQGC) recently reported positive feedback from the FDA for its Phase III programme design for firibastat. There will be two studies required for approval: one focused on efficacy and one on safety. The efficacy study, QGC001/3QG1, will be a three-month 500-patient study comparing firibastat to placebo in difficult-to-treat or resistant hypertension patients who are already on treatments from two or three anti-hypertensive classes. Enrolment is expected to begin by the end of this year with data in H221. The safety study will enrol 750 patients, with 650 staying on the drug for six months and 100 staying on it for a year.
Advanced partnership discussions ongoing
The company has stated it is in advanced discussions with several pharmaceutical companies to partner firibastat and recently announced exclusive negotiations with a potential partner to commercialise firibastat for hypertension in Latin American markets. If these negotiations prove successful, they will enable Quantum Genomics to fund development through non-dilutive financing (we currently model €23.3m in additional financing through the end of 2020.).
QUORUM study in heart failure enrolling patients
The Phase IIb QUORUM study is enrolling 294 subjects from 40 centres in the US and Europe within 72 hours of suffering acute myocardial infarction (AMI), commonly referred to as a heart attack. The primary endpoint will be the change from baseline in the left ventricular ejection fraction (LVEF) after a three-month treatment. Results are expected in H220.
Trial in patients with renal failure launched
Based on analysis of the NEW-HOPE study, firibastat appears to not have any impact on renal function, which is a problem with many popular treatments such as Diovan. To confirm this finding, the company has initiated a small study investigating one 500mg dose of firibastat in 14 healthy volunteers and 14 patients with severe renal failure. Results are expected in April 2020. If confirmed, this finding would help expand the market and provide a marketing edge for firibastat.
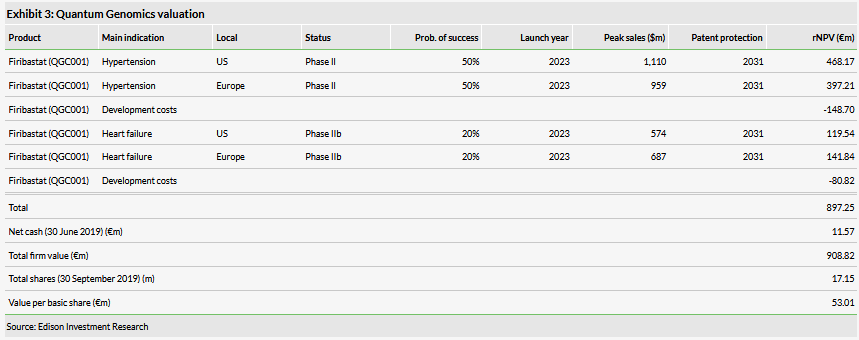
Valuation: €909m or €53.01 per share
We have increased our valuation from €860m or €51.76 per share to €909m or €53.01 per share mainly due to rolling forward our NPV. It was partially offset by a lower net cash balance and a slightly higher number of shares outstanding. Quantum had €11.6m in cash and investments at the end of H119. It has an additional €5.8m available through its equity line of credit with Kepler Cheuvreux.
Business description
Quantum Genomics is a biopharmaceutical company developing firibastat, a brain aminopeptidase A inhibitor for treating hypertension and heart failure. Its mechanism is implicated in the 25% of patients resistant to treatment. The Phase IIb study in hypertension was very positive and a Phase IIb study in heart failure was recently initiated.
Progress on the clinical front
In September Quantum Genomics reported that it received positive feedback from the FDA for its Phase III programme design for firibastat. There will be two studies required for approval: one focused on efficacy and one on safety. The efficacy study, QGC001/3QG1, will be a three-month 500-patient study comparing firibastat to placebo in difficult-to-treat or resistant hypertension patients who are already on treatments from two or three anti-hypertensive classes (firibastat and placebo will be added on top of the current treatment) yet still have systolic automated office blood pressure (AOBP) above 140mmHg. The primary endpoint will be a change from baseline in systolic AOBP. Enrolment is expected to begin by the end of the year with data in H221. The safety study will enrol 750 patients, with 650 staying on the drug for six months and 100 staying on it for a year.
The company’s most recent trial in hypertensive patients, NEW-HOPE, completed enrolment faster than expected, enrolling 256 patients (254 included in the intent-to-treat analysis) in just 10 months. NEW-HOPE focused enrolment on hypertensive overweight (BMI 25–45kg/m2) patients (65% of patients were obese), with a primary endpoint of change from baseline in systolic AOBP at week eight. Patients saw a statistically significant reduction from baseline (p
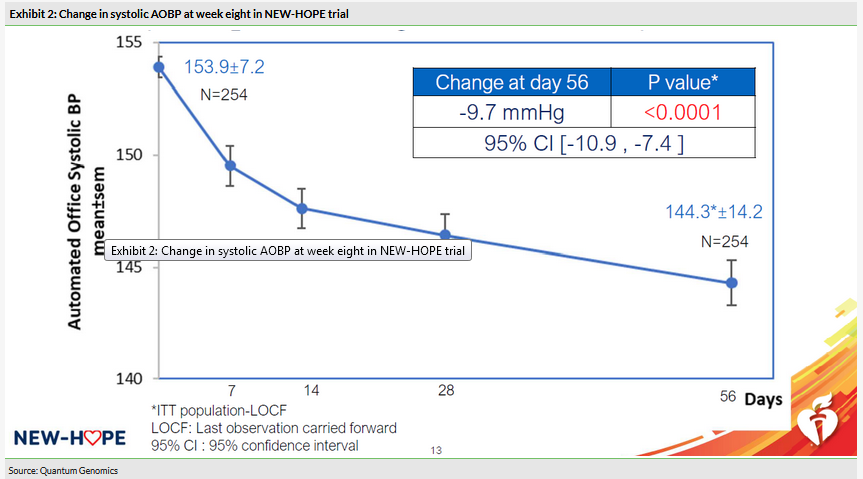
The design of Phase III will be somewhat different than NEW-HOPE with one of the major differences being that patients in NEW-HOPE were taken off their prior treatment (if any) before receiving firibastat, whereas in the upcoming Phase III it will be used on top of the patients’ current treatment regimen. This may make it more difficult to see the degree of treatment effect that was seen in NEW-HOPE. However, another design change that may benefit firibastat is that the QGC001/3QG1 trial will be somewhat longer than NEW-HOPE (90 days versus 56 days) and data from NEW-HOPE indicated a greater efficacy the longer patients were on firibastat (see Exhibit 2).
Importantly, the company has stated it is in advanced discussions with several pharmaceutical companies to partner firibastat and announced in October it is in exclusive negotiations with a potential partner to commercialise firibastat for hypertension in Latin American markets. This potential partner has a portfolio of over 100 products and has signed over 50 international partnerships. Quantum Genomics has also stated it will be moving forward with a regional partnership strategy in hypertension rather than one global partner. This should mean shorter timelines for partnership agreements and an increased commercial focus on firibastat by those smaller regional partners as it would be more strategic for it than for a giant pharmaceutical company.
In terms of the heart failure programme, Quantum Genomics is continuing to enrol the QUORUM study, which will assess the safety and efficacy of Quantum’s drug firibastat compared to ramipril, an angiotensin-converting enzyme inhibitor, in 294 subjects enrolled within 72 hours of suffering AMI, who were treated with primary percutaneous coronary intervention and have reduced LVEF. There are three arms in this randomised, double-blind, active-controlled study with patients receiving either 100mg of firibastat twice a day, 500mg of firibastat twice a day or 5mg of ramipril twice a day. The primary endpoint is the change from baseline in LVEF after a three-month treatment. Secondary endpoints will include cardiac events, functional status and change in heart failure biomarkers. The subjects will be recruited from 40 centres in the US and Europe and trial results are expected in H220. We expect potential partnership discussions for firibastat in heart failure to intensify once the QUORUM study results are out. We do not believe that licensing agreements for hypertension will necessarily preclude separate agreements for heart failure as the product may have different formulations and dosages in the two indications.
Valuation
We have increased our valuation of Quantum Genomics from €860m or €51.76 per share to €909m or €53.01 per share mainly due to rolling forward our NPV. It was partially offset by a lower net cash balance and slightly higher shares outstanding.
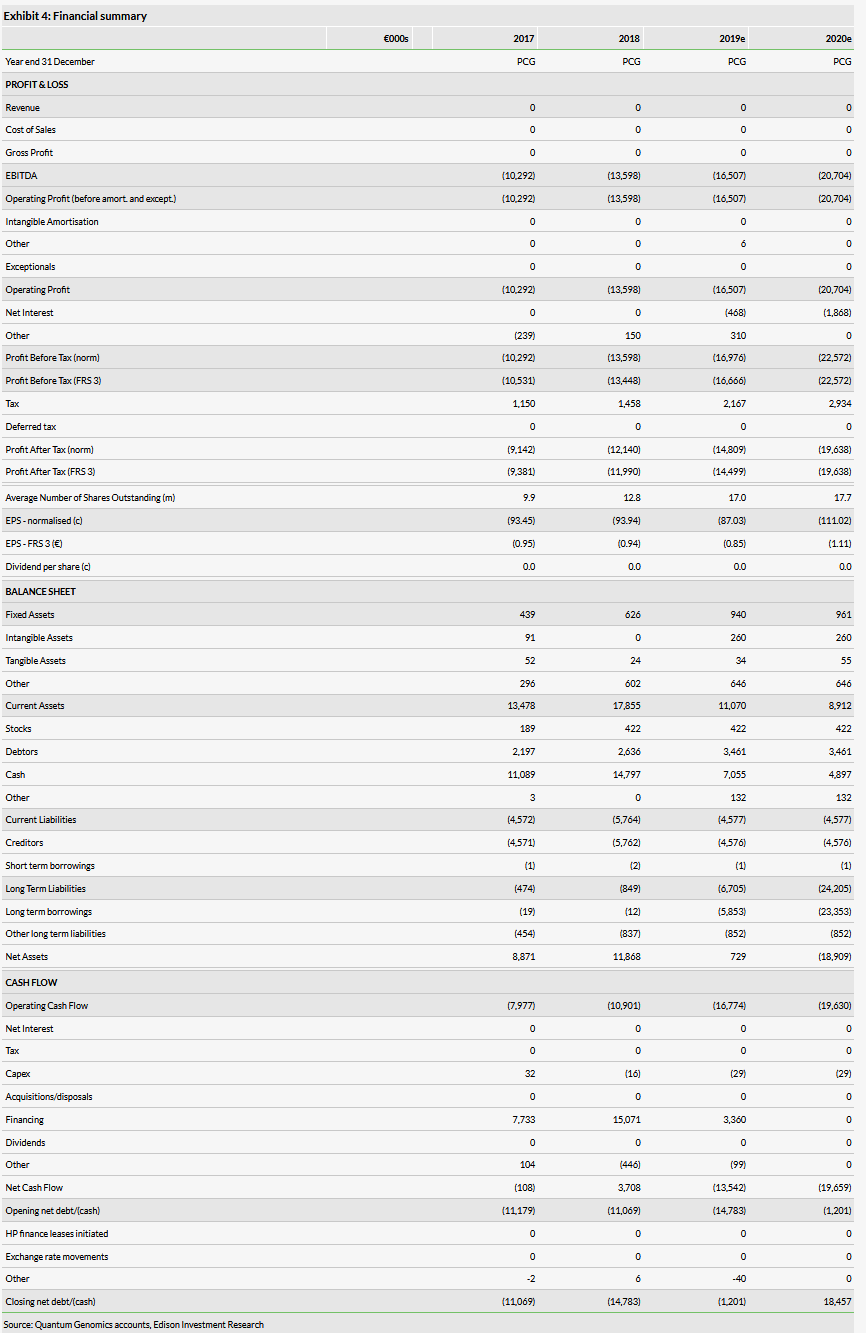
Financials
The company reported an operational loss of €5.3m in H119 compared to €6.8m in H118, with the decrease primarily driven by the completion of the NEW-HOPE study. We have decreased our operating loss estimates for 2019 by around €0.3m to €16.5m due to lower than expected spending in the first half. We have also lowered operating loss estimates for 2020 by €0.2m to €20.7m.
Quantum had €11.6m in cash and investments at the end of H119. In March 2018, it announced an equity line of credit with Kepler Cheuvreux and has approximately €5.8m of the original €24m line remaining after drawing down an additional €3.4m during the first half of the year. The company has stated it believes its available cash and equity line will be enough to fund the company for the next 12 months. We model (as illustrative long-term debt) that the company will use the remainder of the available credit line by the end of 2019 and will raise an additional €17.5m in 2020, assuming no partnerships are signed. These financing needs could be significantly reduced or eliminated through successful partnership discussions and upfront/milestone payments.