QIAGEN N.V. (NYSE:QGEN) has unveiled its latest therascreen FGFR RGQ RT-PCR Kit (therascreen FGFR Kit) in the United States. This kit will be used as a companion diagnostic to identify patients eligible for the treatment with Janssen Biotech’s fibroblast growth factor receptor (FGFR) kinase inhibitor drug, Balversa.
The test will help detect patients with urothelial cancer, whose tumors consist of certain alterations in the fibroblast growth factor receptor 3 (FGFR3) gene.
With this launch, QIAGEN’s molecular diagnostics portfolio is likely to get a substantial boost.
Notably, the company is optimistic about the introduction of this new therascreen FGFR Kit as it is the first companion diagnostic test to have received an FDA approval for spotting FGFR gene alterations. This will help in guiding therapy in any cancer identification.
Urothelial Cancer: A Glimpse
Per the definition provided by Canadian Cancer Society, urothelial cancer or carcinoma (also known as transitional cell carcinoma) is a cancerous tumor of the bladder that can spread (metastasize) to other parts of the body. This type of cancer starts in tissues lining the bladder and other genitourinary organs and is ranked sixth among the most common cancer types in the United States.
According to a recent Grand View research article, this particular type of cancer has been considered the ninth most common malignancy with nearly 2.5 million global patients and 420,000 newly diagnosed cases registered each year.
The global urothelial cancer drug market is currently holding great prospects and is expected to reach $3.6 billion by 2023 at a CAGR of 22.9% from 2018 to 2023.
A View of Therascreen FGFR RGQ RT-PCR Kit
This new diagnostic has been designed to specifically detect the tumors with certain alterations in the fibroblast growth factor receptor 3 (FGFR3) gene. This test will be used alongside QIAGEN’s Rotor-Gene Q MDx automation solution — a kit that examines a patient’s DNA or ribonucleic acid (RNA).
A certain percentage of urothelial carcinoma tumors, having specific FGFR alterations, might be the key drivers of tumor growth. This companion diagnostic by QIAGEN will ensure easy detection of these alterations in the patients eligible for treatment with Balversa. This test will help in the guidance of treatment decisions associated with urothelial cancer and in the process, acknowledge a large percentage of the unmet medical need of the affected patients.
Market Potential
Molecular diagnostics is an area gaining a steady momentum and holding great prospects to experience rapid growth in the coming years. Going by Go Molecular reports, molecular diagnostic tests detect particular sequences in DNA or RNA that may or may not be related to disease and comprise single nucleotide polymorphism (SNP), deletions, rearrangements, insertions, etc.
Per a report by Grand View Research, the global molecular diagnostics market size is anticipated to attain a value of $19.8 billion by 2026 witnessing a CAGR of 9.1% over the forecast period. Rising infections like human papillomavirus and influenza are the primary factors driving the market.
The addition of this diagnostic is expected to broaden QIAGEN’s molecular diagnostic segment, which is one of the four customer classes for sample and assay technologies.
QIAGEN’s Molecular Diagnostics Update
QIAGEN currently provides one of the broadest portfolios of molecular technologies for human healthcare, acknowledging significant market opportunities.
In the context of enhancing this segment, the partnership with Tecan Group to boost the processing of QIAGEN’s QuantiFERON-TB Gold Plus (QFT-Plus) diagnostic test deserves special mention. (Read more: QIAGEN Partners With Tecan to Advance QFT-Plus Diagnostic Test).
Zacks Rank and Share Price Movement
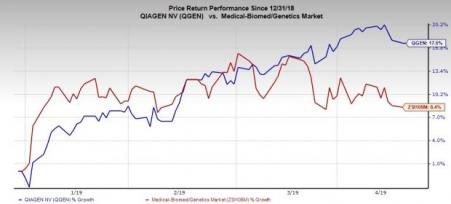
QIAGEN has a Zacks Rank #2 (Buy). Year to date, shares of the company have outperformed its industry. The stock has rallied 17.4% compared with the industry’s growth of 8.4%. The current level is also higher than the S&P 500 Index’s 15.4% increase.
Other Key Picks
Investors interested in other top-ranked stocks from the broader medical space can also consider ANI Pharmaceuticals, Inc. (NASDAQ:ANIP) , Advaxis, Inc. (NASDAQ:ADXS) and DENTSPLY SIRONA Inc. (NASDAQ:XRAY) .
ANI Pharmaceuticals has an earnings growth rate of 30.9% for the next quarter. The company sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Advaxis has an earnings growth rate of 61.8% for the next quarter. The company has a Zacks Rank of 1.
DENTSPLY SIRONA has a long-term earnings growth rate of 9.6%. The company carries a Zacks Rank of 2.
Will you retire a millionaire?
One out of every six people retires a multimillionaire. Get smart tips you can do today to become one of them in a new Special Report, “7 Things You Can Do Now to Retire a Multimillionaire.”
Advaxis, Inc. (ADXS): Free Stock Analysis Report
QIAGEN N.V. (QGEN): Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP): Free Stock Analysis Report
DENTSPLY SIRONA Inc. (XRAY): Free Stock Analysis Report
Original post
Zacks Investment Research
