Pixium Vision SA (PA:PIX) is developing Prima, a potentially breakthrough wireless sub-retinal implant that generates electrical impulses at the retinal bipolar cell level to restore a form of central visual perception. Pixium is on track to start an EU pivotal study for its Prima bionic vision system (BVS) in H219 for the treatment of advanced dry age-related macular degeneration (Dry-AMD) involving geographic atrophy (GA). This follows the release of positive six-month data in January 2019 for its five-patient EU feasibility study. Using a risk-adjusted NPV model, we obtain a pipeline rNPV (including net cash) of €99.5m, vs €99.0m previously.
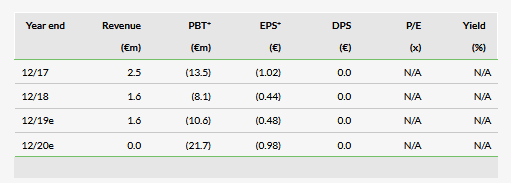
Prima can provide benefit in severe Dry-AMD cases
Prima seeks to address a large unmet market indication, advanced stages of Dry-AMD involving GA. Feasibility data suggests the current Prima iteration provides visual acuity (VA) in the 20/460 to 20/550 range (about 4% of normal vision), which we estimate could potentially provide benefit in a target market of about 63,000 patients in Europe and 49,500 in the US with severe GA stages and lower pre-implantation VA. The level of vision provided by Prima in such cases can potentially enable the recognition of shapes and symbols in patients who may not have been able to identify them before the implantation. Providing such functional benefit may support reimbursement discussions if the product obtains regulatory approval.
EU pivotal study expected to start in H219
Pixium plans to begin discussions with regulators to conduct a pan-European pivotal trial across several countries and multiple centres. Pixium’s goal is to start recruitment for the pivotal study in H219, potentially resulting in initial implantations before YE19. We estimate it will require 12 months of follow-up data within this trial for European regulators to provide CE mark approval. We believe that EU commercialisation (CE mark approval) may occur in H222.
Valuation: €99.5m in equity, or €4.49 per share
We believe Pixium’s cash on hand should be sufficient for it to maintain its operations into Q220. We continue to estimate that Pixium will raise €75m through 2021 to fund Prima development. As per Edison policy, we model these as debt financing. We continue to value Pixium using an rNPV approach, employing a 12.5% cost of capital and applying a 15% probability of success estimate for Prima. Following minor changes to our market size, forex and EU pricing assumptions, we now obtain a pipeline rNPV (enterprise value) of €91.7m, up from €91.2m, previously. After including €7.8m net cash at 31 December 2018, we obtain an equity valuation of €99.5m, or €4.49 per share (compared to €4.50 previously).
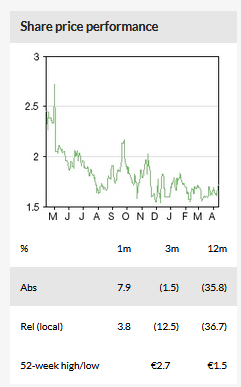
Business description
Pixium Vision develops bionic vision systems for patients with severe vision loss. Its lead product, Prima, is a wireless sub-retinal implant system designed for Dry-AMD. The firm has completed a human feasibility study in Europe and expects to start implantations in a US feasibility study in H119.