This week, companies like Mylan (NASDAQ:MYL) , Allergan (NYSE:AGN) and Teva (NYSE:TEVA) reported earnings results. The spotlight also remained on Roche (OTC:RHHBY) which saw its immuno-oncology drug fail in a pivotal study.
Recap of the Week’s Most Important Stories
A Look at Earnings Results: Mylan, Allergan and Teva all reported first quarter results this week. While Mylan topped earnings estimates, it missed on sales with EpiPen being impacted by increased competition and the presence of a lower priced authorized generic. (Read more: Mylan Beats on Q1 Earnings, Revenues Miss Estimates). Teva’s sales also fell short of expectations (Read more: Teva Q1 Earnings In Line, Sales Lag as Copaxone Falls). The company continues its search for a permanent CEO and has decided to divest its global Women’s Health business as well as the Oncology and Pain business in Europe. Teva expects to generate $1 billion from the sale of these businesses as well as additional assets. Meanwhile, Allergan delivered a “beat and raise” quarter (Read more: Allergan Beats Q1 Earnings Estimates, Ups 2017 View).
Setback for Roche as Tecentriq Fails in Pivotal Study: Swiss pharma giant, Roche, was hit by results from a pivotal study (IMvigor211) on its immuno-oncology drug, Tecentriq, a PD-L1 inhibitor. Tecentriq failed to meet the primary endpoint of overall survival (OS) in the study conducted in second-line bladder cancer patients. Tecentriq had gained accelerated FDA approval for this indication based on duration of response and tumor response rate and IMvigor211 was being conducted to serve as a confirmatory study to support full approval. With results falling short, the drug’s conditional approval for this indication could be at stake though Tecentriq also gained accelerated approval for the first-line patient population.
Meanwhile, other checkpoint inhibitors that gained accelerated approval for this indication based on duration of response will also feel the heat until confirmatory data is out. In this regard, we note that Merck’s (NYSE:MRK) anti-PD-1 therapy, Keytruda, met the primary endpoint of OS in a phase III study -- the data has been submitted for FDA review with a response from the agency expected by Jun 14, 2017.
So far in 2017, Roche has outperformed the Zacks categorized Large Cap Pharmaceuticals industry with shares gaining 16.6% while the industry is up 7.7%. Roche is a Zacks Rank #3 (Hold) stock -- you can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Label Expansion for Merck’s Keytruda: Merck got a boost with the FDA granting accelerated approval to Keytruda for use in combination with Alimta (pemetrexed) and carboplatin (pem/carbo), a commonly used chemo regimen, for the first-line treatment of metastatic nonsquamous non-small cell lung cancer (NSCLC), irrespective of PD-L1 expression.
Novartis’ Kisqali Co-Pack Gets FDA Nod: Swiss drugmaker Novartis gained FDA approval for a combination pack of two of its breast cancer drugs -- the company said that the Kisqali Femara Co-Pack can be used for the treatment of HR+/HER2- advanced or metastatic breast cancer in postmenopausal women. The convenience of obtaining a full 28-day cycle of the two medicines in one package with a single prescription and co-pay could well provide Novartis with a convenience edge. The company said that the pack is priced at the same cost as Kisqali alone. Kisqali is among Novartis’ most recent product launches.
Another Approval for Pfizer/Merck KGaA’s Bavencio: The FDA granted accelerated approval to Pfizer (NYSE:PFE) and Merck KGaA’s immunotherapy drug, Bavencio, for a second indication. The drug has been approved for urothelial carcinoma, an aggressive disease with a high rate of recurrence. Bavencio was approved earlier this year for metastatic Merkel cell carcinoma (mMCC), a rare and aggressive skin cancer.
Pfizer Boosts Gene Therapy Portfolio with Sangamo Deal: In an attempt to boost its rare diseases and gene therapy pipeline, Pfizer has entered into an exclusive, worldwide collaboration and license agreement with Sangamo for the development and commercialization of gene therapy programs for hemophilia A. The deal covers SB-525, one of Sangamo’s four lead pipeline candidates, which is expected to enter the clinic this quarter. The agreement under which Pfizer will pool its expertise in rare disease, gene therapy, and hemophilia with Sangamo’s deep knowledge in genomic therapies will see Sangamo getting an upfront payment of $70 million. Sangamo could get milestone payments of up to $475 million, including up to $300 million related to SB-525 and up to $175 million for additional hemophilia A gene therapy candidates that may be developed under the collaboration. Tiered double-digit royalties on net sales also form a part of the deal.
This is not the first time that Pfizer has signed a gene-therapy focused deal. Last year, the company had acquired Bamboo Therapeutics, a privately held biotech company focused on developing gene therapies for the treatment of patients with certain rare diseases.
AstraZeneca Provides Data on Imfinzi & Asthma Drug: AstraZeneca’s (NYSE:AZN) investigational asthma drug, tralokinumab, failed to meet the primary endpoint of a significant reduction in the annual asthma exacerbation rate (AAER) in the overall population of severe, uncontrolled asthma patients, in the first of two pivotal studies.
The company, however, noted that a clinically-relevant reduction in AAER was observed in a sub-population of patients with an elevated biomarker associated with increased IL-13 activity. This sub-group of patients will now be the focus for the second ongoing pivotal study. Results from the second study are expected in the second half of the year.
Meanwhile, the company said that Imfinzi met a primary endpoint in a study being conducted in patients with locally-advanced, unresectable NSCLC. The planned interim analysis also showed that Imfinzi has a favorable benefit/risk profile.
Performance
Large Cap Pharmaceuticals Industry 5YR % Return
The NYSE ARCA Pharmaceutical Index was down slightly (0.40%) over the last four trading sessions. AstraZeneca was up 2.2% while Lilly’s shares slipped 2.8%. Over the last six months, AstraZeneca was up 11.3% while Bristol-Myers was down 2.2% (See the last pharma stock roundup here: Merck, Pfizer Report Q1 Earnings, J&J Talc Powder Lawsuit).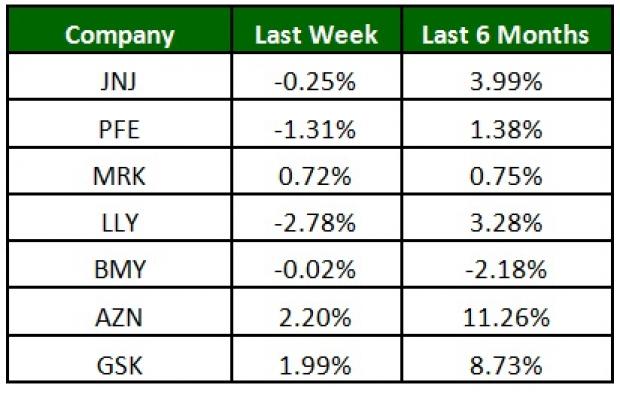
What's Next in the Pharma World?
Watch out for an update from Johnson & Johnson (NYSE:JNJ) regarding its Pharmaceutical businesses next week.
Sell These Stocks. Now.
Just released, today's 220 Zacks Rank #5 Strong Sells demand urgent attention. If any are lurking in your portfolio or Watch List, they should be removed immediately. These sinister companies because many appear to be sound investments. However, from 1988 through 2016, stocks from our Strong Sell list have actually performed 6X worse than the S&P 500.
See today's Zacks "Strong Sells" absolutely free >>.
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Teva Pharmaceutical Industries Limited (TEVA): Free Stock Analysis Report
Mylan N.V. (MYL): Free Stock Analysis Report
Original post
Zacks Investment Research