Pfizer Inc. (NYSE:PFE) and Eli Lilly and Company (NYSE:LLY) announced that a higher dose of their NGF inhibitor, tanezumab, met the primary endpoint in a phase III study, evaluating it in patients with chronic low back pain (“CLBP”).
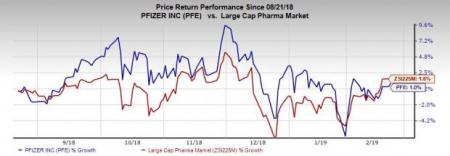
In the past six months, shares of Pfizer have risen 1% compared with the industry’s gain of 1.6%.
The phase III study evaluated two dosages – 5 mg or 10 mg – of tanezumab, a non-opioid candidate, subcutaneously administered every eight weeks for 56 weeks in patients with moderate-to-severe CLBP, followed by 24-week safety follow-up period. The eligible patients experienced inadequate relief or are intolerant to analgesics and suffering from the disease for more than three consecutive months.
Top-line data from the study showed that treatment with the 10 mg dose of tanezumab led to a statistically significant improvement in pain compared to placebo after 16 weeks of treatment. Although the lower dose of 5mg demonstrated numerical improvement in pain compared to placebo, it did not achieve statistical significance. Detailed results from the study will be submitted for future scientific publication and presentation.
The company estimates that there are 8 million patients with moderate-to-severe CLBP in the United States, which represents a significant opportunity for the candidate as currently available therapies do not have adequate patient response.
Apart from CLBP, tanezumab is also being developed for other pain indications, osteoarthritis and cancer pain. The companies have successfully completed two late-stage studies evaluating tanezumab in patients with osteoarthritis pain.
Tanezumab works by inhibiting nerve growth factor, which suppresses pain signal in the body. Its approval will provide a safer pain medication for patients in the United States where opioid abuse is widespread and patients are largely underserved by the existing medications.
We remind investors that Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) and Teva Pharmaceutical Industries Limited (NYSE:TEVA) are also developing a NGF antibody, fasinumab for treating osteoarthritis pain.
In a separate press release, Pfizer announced that the European Commission has granted approval to Zirabev (bevacizumab), its biosimilar version of Roche’s cancer drug, Avastin. Zirabev is approved for the treatment of several cancer indications, for which the reference product is approved like colon cancer, metastatic breast cancer, unresectable advanced, metastatic or recurrent non-small cell lung cancer (“NSCLC”), among others.
This is Pfizer’s second oncology biosimilar to be approved in Europe, the first being Roche’s another cancer drug, Herceptin. Meanwhile, Zirabev is also under review in the United States along with biosimilar versions of Rituxan and Herceptin with FDA decisions on all expected in 2019.
Zacks Rank
Pfizer currently carries a Zacks Rank #4 (Sell).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
Eli Lilly and Company (LLY): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA): Free Stock Analysis Report
Original post