PerkinElmer, Inc. (NYSE:PKI) recently announced that it has received Emergency Use Authorization (EUA) from the FDA for its New Coronavirus RT-PCR test. Clinical laboratories that are certified under Clinical Laboratory Improvement Amendments (CLIA) can start utilizing this test kit to detect SARS-CoV-2 (virus causing COVID-19) immediately.
Notably, this test is marketed as an in-vitro diagnostic (IVD) device on basis of fulfilling the requirements of European In Vitro Diagnostic Directive (IVDD). The test kit is now available in over 30 countries worldwide.
With this test kit, PerkinElmer is likely to boost its Diagnostics business and strengthen presence in the global infectious disease diagnostics market.
Significance of the Approval
With the coronavirus pandemic showing no signs up letting up, the demand for testing is on the rise posing a crucial challenge. The company’s New Coronavirus Nucleic Acid Detection Kit is a real-time RT-PCR test developed for the qualitative detection of nucleic acid from SARS-CoV-2 in human oropharyngeal and nasopharyngeal swab samples.
In terms of flexibility, the test has proven to be compatible with a variety of sample types and performance has been verified with case studies from outbreak’s point of origin.
Per management, the breadth of the company’s total workflow solution positions it to rapidly address the needs of its clinical diagnostics customers.
Coronavirus Outbreak and Its Impact
The coronavirus disease, which was first detected in central China in December, has turned into a full-blown pandemic, and its panoptic impact has left most of the world rattled and shocked. In fact, more than 53,000 people across the United States has been infected with the novel coronavirus with deaths going up to at least 703.
Amid this crisis, some key players have made significant progress with regards to testing in order to check the spread of pandemic.
For example, Becton, Dickinson and Company (NYSE:BDX) , also known as BD, together with CerTestBiotec, recently announced the receipt of CE mark for the VIASURE SARS-CoV-2 real time test adapted for the BD MAX System. Thus, the polymerase chain reaction (PCR) test, utilized for detecting COVID-19, is now available to clinical laboratories.
Also, Abbott Laboratories (NYSE:ABT) announced the receipt of the Emergency Use Authorization (EUA) from the FDA to use its molecular test RealTime SARS-CoV-2 EUA to detect the novel coronavirus (COVID-19). Notably, the test will run on the company’s m2000 RealTime System.Abbott is on track to ship 150,000 RealTime SARS-CoV-2 EUA tests to existing customers in the United States. At the same time, the company will coordinate with health systems and government authorities to supply additional m2000 systems per requirement.
Market Prospects
Per a report published on Grand View Research, the global molecular diagnostics market was valued at $9.2 billion in 2019 and is expected to reach $18.2 billion by 2027, witnessing a CAGR of 9% between 2020 and 2027. Factors like technological advancements in molecular diagnostics and the rising prevalence of infectious diseases are likely to drive the market.
Hence, the latest development has been a well-timed one for PerkinElmer.
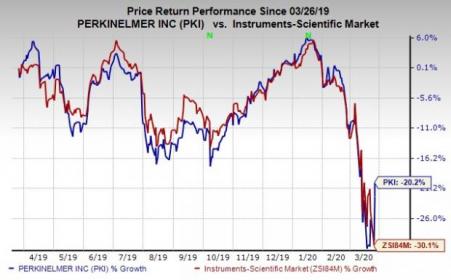
Price Performance
Over the past year, shares of this Zacks Rank #3 (Hold) company have lost 20.2% compared with the industry’s decline of 30.1%.
A Key Pick
A better-ranked stock from the broader medical space is The Cooper Companies, Inc. (NYSE:COO) , which currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Cooper Companies has a projected long-term earnings growth rate of 10.8%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Becton, Dickinson and Company (BDX): Free Stock Analysis Report
PerkinElmer, Inc. (PKI): Free Stock Analysis Report
The Cooper Companies, Inc. (COO): Free Stock Analysis Report
Abbott Laboratories (ABT): Free Stock Analysis Report
Original post
Zacks Investment Research
