Novo Nordisk (CO:NOVOb) A/S (NYSE:NVO) announced that the Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) of the FDA voted 17-2 in favor of Victoza that the drug provides substantial evidence of cardiovascular risk reduction in patients with type II diabetes. The positive panel recommendation bring Novo Nordisk a step closer to get the cardiovascular indication included on the label of its key top-line driver Victoza.
Victoza is a once-daily human glucagon-like peptide-1 (GLP-1) analogue approved for adult type II diabetes. The drug is one of the main revenue contributors for the company.
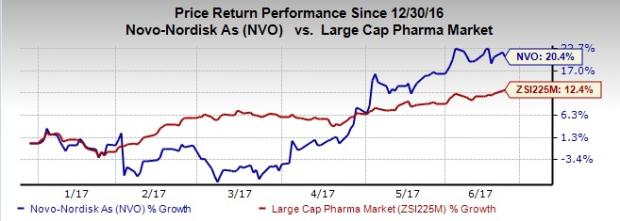
Novo Nordisk’s share price shows that the company has outperformed the Zacks classified Large Cap Pharmaceuticals industry year to date. The stock rallied 20.4% compared with the industry’s gain of 12.4%.
We note that the Advisory Committee’s favorable vote was based on data from the LEADER cardiovascular outcomes trial, which involved more than 9,300 people with type II diabetes at high risk of major cardiovascular events. The study evaluated the cardiovascular benefit of Victoza over a period of up to 5 years in. The study compared the addition of either Victoza or placebo to the standard of care, with the Victoza arm meeting the primary endpoint of non-inferiority. Results also demonstrated superiority of Victoza in a statistically significant reduction of cardiovascular risk.
As instances of death due to cardiovascular diseases are significantly higher in adults with diabetes compared with those without diabetes, the addition of positive cardiovascular outcomes on the label of the diabetes drug can give sales a boost.
The supplemental new drug application for Victoza to include the cardiovascular indication was submitted to the FDA in October 2016 and regulatory feedback in the US is expected in the third quarter of 2017.
We remind investors that Eli Lilly and Company (NYSE:LLY) received FDA approval last year to include cardiovascular risk reduction data from the EMPA-REG OUTCOME study on the label of Jardiance. The updated label including the cardiovascular indication was launched in Jan 2017 while the American Diabetes Association (ADA) has also updated its diabetes treatment guidelines. The European Commission also okayed the Jardiance label update for the cardiovascular indication in 2016.
However, in Apr 2017, Merck & Co., Inc. (NYSE:MRK) was denied approval by the FDA to include cardiovascular outcomes data from the TECOS study on the labels of its DPP-IV inhibitor Januvia (sitagliptin) and other medicines containing Januvia.
AstraZeneca’s (NYSE:AZN) Bydureon also failed to reduce cardiovascular risk in a phase IIIb/IV cardiovascular outcomes study EXSCEL.
Zacks Rank
Novo Nordisk currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors. Click here for Zacks' secret trade>>
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Novo Nordisk A/S (NVO): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research