Swiss pharma giant Novartis AG (NYSE:NVS) announced that the European Commission (EC) has approved its multiple sclerosis drug, Mayzent (siponimod).
Mayzent is a sphingosine 1-phosphate receptor modulator that selectively binds to S1P1 and S1P5 receptors.
The drug is approved for the treatment of adult patients with secondary progressive multiple sclerosis (SPMS), with active disease evidenced by relapses or imaging features of inflammatory activity.
The approval was based on data from the randomized, double-blind, placebo-controlled EXPAND study, which compared the efficacy and safety of Mayzent with that of placebo in a broad range of SPMS patients. Results showed that the drug significantly reduced the risk of disease progression, including physical disability and cognitive decline.
The drug was approved in the United States in March 2019 for the treatment of relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome (CIS), relapsing remitting disease and active secondary progressive disease, in adults.
Approval in additional geographies will strengthen Novartis’ MS portfolio, which comprises approved drugs like Gilenya and Extavia (interferon beta-1b for subcutaneous injection). Notably, Extavia is approved in the United States for the treatment of RMS. The drug is approved in Europe to treat patients with RRMS and SPMS with active disease.
Apart from the approved drugs, Novartis has a promising candidate, ofatumumab, in its pipeline. Ofatumumab (OMB157), a fully human anti-CD20 monoclonal antibody (mAb) targeting B-cells, is in development for treating RMS. The drug met primary endpoints to reduce the ARR in patients with RMS in a phase III study. If approved, ofatumumab will potentially become a treatment for a broad RMS population and the first subcutaneous B-cell therapy that can be self-administered at home.
Novartis’ generic division, Sandoz, markets Glatopa, which is a generic version of Teva Pharmaceuticals’ (NYSE:TEVA) Copaxone (both 20 and 40 mg), in partnership with Momenta Pharmaceuticals (NASDAQ:MNTA) .
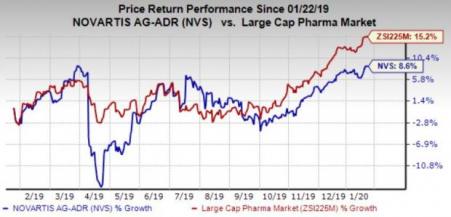
Novartis’ shares have gained 8.6% in the past year compared with the industry’s growth of 15.2%.
However, competition is stiff in the MS market. Notably, Biogen, Inc. (NASDAQ:BIIB) holds a strong position in this market, with a wide range of products, including Avonex, Tysabri, Tecfidera and Plegridy.
Novartis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Novartis AG (NVS): Free Stock Analysis Report
Biogen Inc. (BIIB): Free Stock Analysis Report
Momenta Pharmaceuticals, Inc. (MNTA): Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA): Free Stock Analysis Report
Original post
Zacks Investment Research
