A timely raise
NovaBay Pharmaceuticals Inc (AMEX:NBY) has raised $6.7m from the sale of 5.6m shares at $1.20 per share, a modest 5% discount to the previous close. We estimate end-Q114 net cash at $15m. With the option to supplement reserves through a ~$4m ATM facility with Ascendiant Capital, NovaBay has financial flexibility ahead of important clinical data in mid-2014. Results from a global 450-patient Phase IIb study of its lead anti-infective agent, auriclosene, in viral conjunctivitis are due and positive data could re-rate the stock. Post-issue our valuation rises to $90m (vs $80m), or $1.76 (vs $1.75) per share.
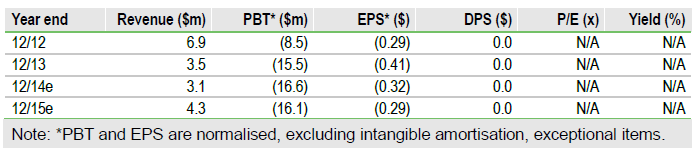
Fresh funds
NovaBay issued 5.6m shares at $1.20 per share, for gross proceeds of $6.7m ($6.1m net). The offering included 18-month warrants to purchase up to 1.4m shares at an exercise price of $1.56 per share. There are now 6.2m warrants outstanding (strike prices: $1.33 to $3.75; expiry dates: August 2014 to July 2016) which could raise up to $10.4m. We estimate end- Q114 cash of $15m, post issue, based on $13m at Q413, $4.5m cash burn in Q114 and $0.5m from the Ascendiant ATM.
Approaching a major inflection point
The 450-patient viral conjunctivitis study is the biggest trial conducted so far with auriclosene. The trial is using an FDA-approved point-of-care adenoviral screening test (AdenoPlus) to ensure correct patient enrolment (ie exclude bacterial conjunctivitis). The company expects that approximately two-thirds of patients will have a more severe, sight-threatening form called epidemic keratoconjunctivitis (EKC). A previous Phase II study observed improvements in microbiological success, sustained clinical cure and blurred vision in the EKC patient subset.
Validating data required
These results are important in the context of prior setbacks with auriclosene in what NovaBay believes were sub-optimal Phase II studies in viral conjunctivitis (run by former partner Alcon; small number of adenovirus positive patients) and impetigo (conducted by Galderma; sub-optimal formulation). NovaBay plans to initiate further clinical studies with auriclosene in 2014, with fresh Phase II trials for urinary catheter blockage and encrustation (UCBE) and impetigo.
Valuation: Adjusted to $90m, $1.76 per share
Our sum-of-the-parts DCF valuation rises to $90m (vs $80m), or $1.76 (vs $1.75) per share, to reflect the new share issue and an increase in our near-term sales estimates for NeutroPhase. This is fair value for the stock ahead of key catalysts, particularly the Phase IIb data for auriclosene in viral conjunctivitis that could re-rate the stock and provide options to secure fresh finance and/or new partnerships.
To Read the Entire Report Please Click on the pdf File Below