Roche’s recent decision to advance rontalizumab, their anti-interferon-alpha (IFNα) antibody, into Phase III trials for lupus suggests that clinical proof-of-concept has been achieved in Phase II trials. This lends validation to Neovacs’s (OLW.F) IFNα-Kinoid, which has completed a Phase I/II trial for lupus with encouraging efficacy data. Neovacs is currently planning a Phase IIb trial in lupus. Separately, it is planning a Phase IIb for the TNFα-Kinoid in rheumatoid arthritis (RA) and is expecting final results of a Phase IIa study in Crohn’s Disease (CD). Additional positive Phase II trial data could attract a licensing partner.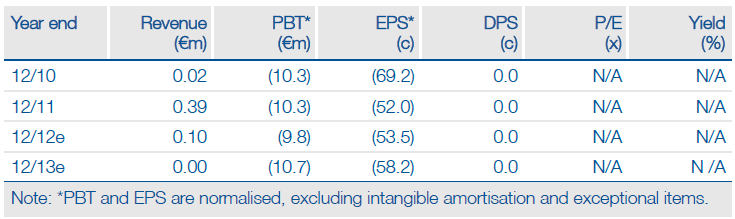
IFNα-Kinoid is competitive in a changing landscape
Roche recently announced that rontalizumab, their humanised monoclonal antibody (mAb) that targets interferon-alpha (IFNα), would be progressing into Phase III trials. This is the first anti-IFNα mAb to reach the Phase III trials stage, which helps to validate the IFNα targeting approach for lupus, including Neovacs’s IFNα-Kinoid.
A Phase I/II trial for lupus has completed
In November 2011, a Phase I/II randomised, double-blind, placebo-controlled trial of IFNα-Kinoid administered in 28 mild-to-moderate lupus patients demonstrated that all the treated patients produced anti-IFNα antibodies and the higher doses had a statistically significant impact on the interferon and lupus signatures. IFNα-Kinoid was also well tolerated and had a good safety profile. A Phase IIb trial is currently being planned.
Financials: Funded to H113
Neovacs ended H112 with cash of €6.6m, which should provide a sufficient runway to complete the CD Phase IIa trial. Neovacs received an OSEO-Anvar grant of €99k for the IFNα-Kinoid in H112. Our financial model suggests that Neovacs will need to raise capital in Q113.
Valuation: Risk-adjusted NPV of €101m
Neovacs has a current market cap of €28m and cash of €6.6m, resulting in an EV of €21m. By comparison, we calculate a risk-adjusted NPV of €101m based on prudent assumptions of the two products’ probability of success in each indication, launch date, pricing and market penetration.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Neovacs Update: Changing Lupus Landscape
Published 10/09/2012, 08:16 AM
Updated 07/09/2023, 06:31 AM
Neovacs Update: Changing Lupus Landscape
Interferon with lupus
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.