Seeding discovery and MOR103 set to flourish
A key goal of Morphosys (XETRA:MORG) this year is to deepen and broaden its proprietary pipeline. This has led to the formation of a discovery collaboration with Temple University to obtain antibodies against innovative therapeutic targets. MorphoSys also aims to in-license products at around the IND stage of development to fill the gap between its discovery and clinical programmes. In the meantime, data on AstraZeneca’s mavrilimumab suggest that MOR103 could be competitive against TNF inhibitors in rheumatoid arthritis (RA). We increase our valuation by €233m to €1.83bn.
Temple University alliance to seed pipeline
MorphoSys has signed an alliance agreement with The Moulder Center for Drug Discovery Research at Temple University, under which the company will give the Moulder Center access to the Ylanthia technology and receive an exclusive option to develop any resulting antibodies. This should enable MorphoSys to gain access to innovative targets and expand its early drug discovery pipeline.
Key focus of management to grow pipeline
We expect MorphoSys to sign more deals in FY14 to expand its pipeline, as management has indicated that broadening its proprietary portfolio is a key objective after the licensing deals with GSK and Celgene in FY13. As well as other early discovery alliances, it is likely that MorphoSys will in-license products at the preclinical/Phase I stage as it did with MOR208, which was developed by Xencor.
Potential of MOR103 highlighted
In a Phase IIb study (n=326) in RA, 73.4% of patients receiving ‘high dose’ mavrilimumab achieved an ACR20 response compared to 24.7% of patients on placebo. MOR103 and mavrilimumab have similar mechanisms of action, targeting GM-CSF and its receptor respectively. This data suggest that both antibodies could have a favourable efficacy profile in comparison with the anti-TNF therapies, which generate revenues of >$25bn. GSK, which licensed MOR103 from MorphoSys in June 2013, is expected to initiate a Phase IIb study in the next few months.
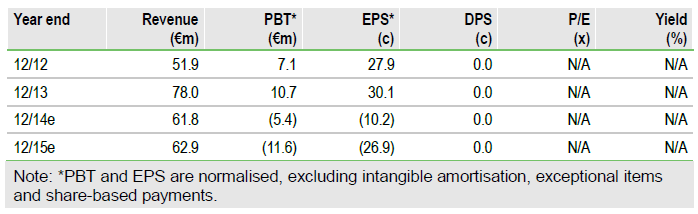
Valuation: DCF valuation of €1.83bn
We raise our valuation by €233m to €1,831m (€69.85/share). We double potential peak sales of MOR103 in RA to €2.7bn due to data on mavrilimumab and increase the likelihood of success for J&J’s guselkumab by 20% to 50%, due to impressive Phase IIb psoriasis data. After the Q113 results, we slightly revise our estimates, which had been amended in the March Edison Insight following the FY13 results.
To Read the Entire Report Please Click on the pdf File Below