The initial data from the Phase Ib/IIa trial in rheumatoid arthritis (RA) with MOR103 increase the likelihood that MorphoSys can partner the product ahead of the Phase Ib trial in multiple sclerosis (MS). In the study, a strong efficacy signal with early onset of action was detected at all doses tested and the product was well tolerated, suggesting that MOR103’s activity compares favourably to that of RA treatments. More data from the study could be reported at the ACR meeting in November. We have raised our valuation from €659m to €697m.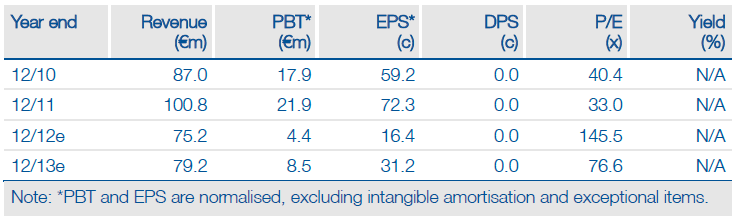
Impressive Initial Data On MOR103
Initial results from the Phase Ib/IIa trial in RA with MOR103, which inhibits GM-CSF activity, showed that at the ACR20 score improvements at week four compared to placebo were 17.6 with dosing of 0.3mg/kg, 60.8 with 1.0mg/kg and 23.0 with 1.5mg/kg. These efficacy levels are similar or better than that seen in clinical trials with the main approved treatments for RA. MOR103 was also well tolerated at all doses.
More Data Expected In Q412
MorphoSys is submitting a late-breaking abstract for the ACR meeting in November, where it hopes to present more results. During the Phase Ib/IIa trial, other efficacy scores (ACR50, DAS28, EULAR) and changes in biomarkers were measured, and MRI analysis was conducted. Phase Ib data with a subcutaneous formulation should be reported soon, the Phase Ib/IIa trial used intravenous administration.
Increased Prospect Of Major Licensing Deal
MorphoSys aims to partner MOR103 with the data from this trial. Despite the promising data, it is not a foregone conclusion as RA is a very competitive space with over 30 biologic products on the market or in late-stage clinical trials. However, the strength of the data, the fact that MOR103 has three mechanisms of action (most only have one) and its potential in MS (Phase Ib data expected in H213) count in its favour. Two reference deals for a partnering are Abbott’s in-licensing of tregalizumab from Biotest ($480m deal) and of GLPG0634 from Galapagos (>$1bn deal).
Valuation: DCF Valuation Of €697m
We have increased our valuation by €38m to €697m, mainly because of the Phase Ib/IIa results. We have revised estimates and MorphoSys remains on track to achieve FY12 revenue guidance of €75-80m, but it will need to earn significant milestones or sign major alliance deals in Q412 as no milestones or deals were announced in Q312.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
MorphoSys Clinical Trial Results
Published 10/05/2012, 10:35 AM
Updated 07/09/2023, 06:31 AM
MorphoSys Clinical Trial Results
MOR Prospects Of A Deal
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.