Shares of Momenta Pharmaceuticals Inc. (NASDAQ:MNTA) gained 1.8% on Aug 2 even though the company reported a wider-than-expected loss and missed revenue estimates in the second quarter.
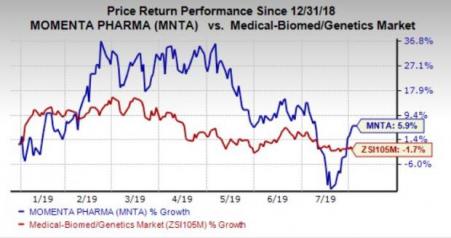
Momenta’s stock has gained 5.9% in the year so far, against the industry’s decline of 1.7%.
The company reported a loss of $1.16 per share in the quarter, wider than the Zacks Consensus Estimate of a loss of 42 cents and the year-ago quarter’s loss of 91 cents.
Revenues in the quarter came in at $5.2 million, which declined from $13.0 million in the year-ago quarter and missed the Zacks Consensus Estimate of $9 million.
Quarter in Detail
Momenta’s top line comprises product revenues of $3.3 million from Sandoz’s sales of Glatopa, a generic version of Copaxone (20 mg), which decreased from $11.8 million in the year-ago quarter due to increasing competition from Mylan’s (NASDAQ:MYL) entry into the Copaxone market.
Research and development revenues came in at $1.8 million compared with $1.3 million in the year-ago quarter.
Research and development expenses came in at $32.1 million, up from $31.3 million in the year-ago quarter, due to lower personnel costs.
General and administrative expenses were $46.6 million, up 107% year over year due to $21.0 million paid to Amphastar in June 2019.
Pipeline Update
Momenta’s novel auto-immune portfolio includes M230, a Selective Immunomodulator of Fc receptors (SIF3); Nipocalimab (M281), an anti-FcRn monoclonal antibody; and M254, a hypersialylated immunoglobulin designed as a high potency alternative for intravenous immunoglobulin (IVIg).
Momenta recently commenced an adaptive phase II/III clinical study of nipocalimab in warm autoimmune hemolyticannemia (wAIHA). The FDA accepted its Investigational New Drug (IND) application for the same and granted it Fast Track designation. Clinical sites are currently being activated and patient recruitment is underway. Momenta expects to report top-line data from this study by the end of 2021. Vivacity-MG, the phase II study of nipocalimab in generalized myasthenia gravis (gMG), continues to open sites and enroll patients. The top-line data is expected in the second or third quarter of 2020.
Unity, the phase II study of nipocalimab in hemolytic disease of the fetus and newborn (HDFN), continues to open sites and enroll patients. Top-line data is expected in 2021. The candidate has been granted Fast Track designation by the FDA for this indication.
In January 2019, Momenta announced that the first subject was dosed in the phase I/II clinical trial of M254 in immune thrombocytopenia (ITP). The multi-part study has completed Part A, which evaluated M254 in a single ascending dose (SAD) cohort of healthy volunteers, and has advanced into Part B, which will evaluate M254 in a SAD cohort of ITP patients. Parts C and D include a randomized cross-over study comparing M254 to IVIg and a multiple ascending dose (MAD) study of M254, respectively. Enrollment for this trial is ongoing and preliminary data is expected in the first half of 2020.
The phase I trial on M230 in healthy volunteers to evaluate the safety and tolerability is ongoing. Momenta’s partner, CSL, expects to complete the phase I study by the end of 2019.
Momenta has ceased development of M923, a fully-owned proposed biosimilar to AbbVie’s (NYSE:ABBV) Humira, due to changes in the market opportunity associated with the drug’s patent litigation settlements. Meanwhile, M710, which is a proposed biosimilar to Regeneron’s (NASDAQ:REGN) Eylea, is being developed in collaboration with Mylan.
2019 Guidance
Momenta expects 2019 expenses to be $45-$55 million.
Our Take
The company’s second-quarter results were dismal. The generic business has been adversely impacted by competition. Momenta has decided to focus on the discovery and development of novel drugs to treat rare, immune-mediated disorders. The generic business is no longer a key area for the company. The company has narrowed its focus on its biosimilars portfolio.
Hence, Momenta is now focussing on its novel drugs portfolio. We expect investors to focus on further updates from the drug candidates in development as the year progress.
Zacks Rank
Momenta currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
AbbVie Inc. (ABBV): Free Stock Analysis Report
Momenta Pharmaceuticals, Inc. (MNTA): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Mylan N.V. (MYL): Free Stock Analysis Report
Original post
