Merck's (NYSE:MRK) shares gained more than 5% on Jan 16, after the company announced that its anti-PD-1 therapy, Keytruda, in combination with Eli Lilly’s (NYSE:LLY) Alimta (pemetrexed) and carboplatin (pem/carbo) met dual primary endpoints in a confirmatory phase III KEYNOTE-189 study. The combination was evaluated for the first-line treatment of patients with metastatic non-squamous non-small cell lung cancer (NSCLC).
Notably, in May 2017, this combination therapy was granted an accelerated approval by the FDA for the aforementioned indication. Hence, the positive readouts from the phase III confirmatory study will help the company gain a continued approval for the combo therapy.
We remind investors that in the third quarter of 2017, the company mentioned that it is including the overall survival (OS) as a co-primary endpoint in the KEYNOTE-189 study of Keytruda in first-line NSCLC, which will defer the readout from the study to 2019. Therefore, the data now released came in much earlier than investor’s expectations, which is a big boost to the company.
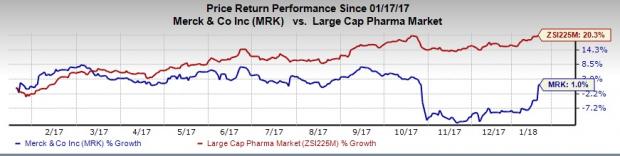
Merck’s shares have inched up 1% in a year’s time, comparing unfavorably with the 20.3% rally of its industry.
The study was designed to evaluate the combination therapy compared with the chemotherapies alone. The study met both its primary endpoints, progression-free survival (PFS) and OS (co-primary endpoint).
Data from the study demonstrated significant improvement in OS and PFS in NSCLC patients receiving Keytruda combo therapy compared with those receiving chemotherapy alone. This was based on an interim analysis conducted by the independent data monitoring committee. It is important to note that Keytruda is the first PD1 inhibitor in combination to show OS in patients with NSCLC.
The company said that data from the trial will be presented at an upcoming medical meeting and submitted to regulatory authorities.
Significantly, in October last year, Merck also announced that it has withdrawn its European regulatory application, which sought an approval for the combination of Keytruda with Alimta and carboplatin for NSCLC. The application was based on positive data from the cohort G of KEYNOTE-021 trial. However, with this new positive readout, possibility of the drug getting a nod from the EU increases.
Note that Roche (OTC:RHHBY) recently presented data on Tecentriq in novel combinations across a broad range of tumors including lung cancer.
We remind investors that Keytruda is the first anti-PD-1 therapy to gain an FDA approval and is being studied for more than 30 types of cancer in above 650 studies including 400 plus combination trials. Keytruda fetched in global sales of $2.5 billion in the first nine months of 2017, up 173% year over year.
Zacks Rank & Key Pick
Merck carries a Zacks Rank #3 (Hold). A better-ranked stock in the health care sector is Exelixis, Inc. (NASDAQ:EXEL) , sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Exelixis’ earnings per share estimates have been revised upward from 72 cents to 73 cents for 2018 over the last 60 days. The company pulled off a positive earnings surprise in each of the trailing four quarters with an average beat of 572.92%. Share price of the company has surged 66.7% in a year’s time.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research