Jazz Pharmaceuticals plc (NASDAQ:JAZZ) announced positive efficacy results from studies evaluating its lead pipeline candidate, JZP-110, in adult patients with excessive sleepiness (ES) associated with narcolepsy and obstructive sleep apnea (OSA). The data were presented at the 31st Associated Professional Sleep Societies (APSS) Annual SLEEP Meeting in Boston, MA.
JZP-110 is being evaluated in a phase III program, treatment of OSA and Narcolepsy Excessive Sleepiness (TONES), for sleep disorder. It comprises four studies.
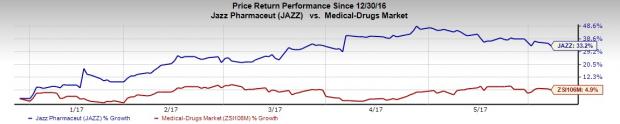
Jazz Pharma’s shares have traded above the Zacks classified Medical-Drugs industry so far this year. Specifically, the company’s shares gained 33.2% while the industry registered an increase of 5.2%.
The parallel group TONES 2 study is evaluating JZP-110 for change in mean sleep latency on the Maintenance of Wakefulness Test (MWT) and in the Epworth Sleepiness Scale (ESS) score from baseline to week 12, in patients with narcolepsy. An equal number of patients were administered with 300 mg, 150 mg, and 75 mg of JZP-110 and a placebo.
Patients administered 150 mg and 300 mg doses showed significantly increased mean sleep latency and a significant decrease in ESS scores for all doses in comparison to placebo. However, statistical significance was reached only for the ESS endpoint.
The TONES 3 study is evaluating JZP-110 for change in mean sleep latency on MWT and ESS score from baseline to week 12 in patients with OSA. TONES 4 was a six week study consisting of two-week flexible-dose titration phase, followed by two-weeks of stable dose treatment for the same indication.
The data from the TONES 3 study administering JZP-110 at 300 mg, 150 mg, 75 mg, and 37.5 mg dosage and a placebo in patients at random showed statistically significant improvements in both the primary endpoints, for all doses when compared to placebo. Patients administered with 150 and 300 mg doses of JZP-100 demonstrated an increase of more than 10 minutes in mean MWT sleep latency and a decrease of 7 points in ESS score at week 12.
The data from TONES 4 study showed a decrease of one minute in mean MWT sleep latency for JZP-110 compared to 12.1 minutes for placebo, at week 6 with week 4 data as baseline. Moreover, the mean ESS score for JZP-110 decreased by 0.1 minutes whereas it increased by 4.5 minutes for placebo.
JZP-110 is also being evaluated in phase II study for ES in Parkinson’s disease.
We note that JZP-110 enjoys Orphan Drug designation in the U.S. for narcolepsy. Also, with the completion of enrolment of patients in all the studies of TONES phase III program, the company is planning to file a new drug application (NDA) to the FDA in late 2017. The company’s marketed drug, Xyrem, is the only FDA approved product for both cataplexy and EDS. It recorded sales of $272.3 million in the first quarter of 2017.
However, there are a number of marketed branded and generic products for treating OSA and narcolepsy including Novartis AG’s (NYSE:NVS) Ritalin-SR.
Zacks Rank
Jazz Pharma currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the health care sector include VIVUS, Inc. (NASDAQ:VVUS) and Zoetis Inc. (NYSE:ZTS) . VIVUS sports a Zacks Rank #1 (Strong Buy) while Zoetis carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates narrowed from 50 cents to 39 cents for 2017 over the last 60 days. The company posted positive earnings surprises in all the four trailing quarters with an average beat of 233.69%.
Zoetis’ earnings per share estimates increased from $2.32 to $2.34 for 2017 over the last 60 days. The company posted positive earnings surprises in each of the four trailing quarters, with an average beat of 9.82%.
3 Stocks to Ride a 588% Revenue Explosion
At Zacks, we're mostly focused on short-term profit cycles, but the hottest of all technology mega-trends is starting to take hold...
By last year, it was already generating $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for those who make the right trades early.
See Zacks' Top 3 Stocks to Ride This Space >>
Novartis AG (NVS): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Jazz Pharmaceuticals PLC (JAZZ): Free Stock Analysis Report
Zoetis Inc. (ZTS): Free Stock Analysis Report
Original post
Zacks Investment Research