We are initiating research on Jaguar Health Inc (NASDAQ:JAGX), which is centered on the development and marketing of its drug crofelemer for diarrheal disorders. It is approved and marketed under the name Mytesi for non-infectious diarrhea in HIV patients receiving anti-retroviral therapy (ART) and drove almost all of the $4.4m in sales in 2018. The company hopes to expand to other indications, aims to initiate a pivotal study for targeted cancer therapy-related diarrhea (CTD) and plans to leverage results from an ongoing Phase II investigator-sponsored study. We initiate with a valuation of $86.5m or $4.14 per diluted share.
Consistency, not flow
Mytesi is one of only two drugs approved by the FDA under its botanical product guidance. It is an extract of the Croton lechleri tree found in the Amazon (NASDAQ:AMZN) and inhibits cystic fibrosis transmembrane conductance regulator (CFTR) and the calcium dependent chloride channel (CaCC), which are responsible for controlling the quantity of water in the bowel, thus resulting in more formed stool. Importantly, this inhibition does not inhibit peristalsis unlike other available anti-diarrheal medicines such as Lomotil and loperamide. The lack of significant constipation risk positions it positively for chronic use. Other indications that the company is exploring include irritable bowel syndrome, inflammatory bowel disease, congenital diarrheal disorders, idiopathic diarrhea and acute infectious diarrhea.
Unmet need but also underappreciated
The drug has a long development history dating to the 1990s and was previously marketed for HIV-associated diarrhea by Salix (launched in 2013) at the time it was bought by Valeant (now Bausch Health). Diarrhea in people with HIV is under reported and under recognized as a significant contributor to poor quality of life in the 1.1 million HIV patients in the US. With the introduction of newer antiretroviral drug therapy, there has been a reduction in the incidence and/or severity of diarrhea, but clinical trial data suggest a continued diarrhea prevalence of 19%.
Valuation: $86.5m or $4.14 per diluted share
We arrive at an initial valuation of $86.5m or $4.14 per diluted share based on a risk adjusted NPV analysis, with the value driven primarily by Mytesi for ART. We assume this program can achieve $74.1m in peak sales if it can capture 12% of the market by 2031. We expect the company to require $80m in additional capital to reach profitability in 2024, which poses significant financing risks to the company if it is not able to secure alternative funding or constrain spending.
Business description
Jaguar Health is a pharmaceutical company marketing Mytesi (crofelemer) for diarrhea associated with HIV treatment. Additionally, the company is developing the drug for a range of other diarrhea indications and has developed a set of products using the drug for animal use.
Investment summary
Company description: A GI-focused drug
Jaguar is a biopharma company focused on marketing and developing the drug Mytesi (crofelemer) for the treatment of diarrheal disorders in the US. Crofelemer is a botanical drug extracted from the Croton lechleri tree that normalizes fluid content in the gut in patients with diarrhea. It is approved and marketed for symptomatic relief of non-infectious diarrhea associated with HIV antiretroviral therapy (ART) in adults, and is being investigated for a range of other causes of diarrhea, the most advanced program being for CTD, which is in a Phase II investigator-initiated trial. The company had $4.4m in sales in 2018, almost entirely from Mytesi.
Valuation: Initiated at $86.5m or $4.14 per diluted share
We arrive at an initial valuation of $86.5m or $4.14 per diluted share based on a risk adjusted NPV analysis. We include the ART program, the CTD program, Canalevia (a canine product for chemotherapy induced diarrhea) and unallocated costs in our model, with ART driving the majority of the valuation ($66.8m). We forecast $74.1m in peak sales in 2031 for the product if it can capture 12% of the market. We may add other indications to our model if the company commits capital to further developing them.
Financials: In the process of converting debt
The company is revenue generating with $1.7m reported for Q219 and had a net loss of $16.7m. These losses are driven in large part by non-cash charges associated with the recapitalization of the company and an undisclosed $4m impairment on the company’s intangible assets. As part of this multi-pronged recapitalization process the company has issued 10m common shares (or equivalent in convertible preferred shares) and 19m warrants (at $2.00) on a base of 0.9m split adjusted shares at the beginning of 2019. The company may substantially dilute further both to eliminate its remaining debt ($7.8m in exchange notes) and to finance operations. We expect the company to require $80m in additional capital to reach profitability in 2024: $30m each in 2020 and 2021, and $20m in 2022. We forecast that this is likely to be difficult using equity at the current market capitalization.
Sensitivities: Marketing and clinical challenges
The company’s operations are focused primarily on marketing Mytesi for ART diarrhea, which poses a number of significant hurdles. It is more difficult for a small company like Jaguar to market a single drug because of limited resources, whereas a larger pharma company could simply add another asset to its offering. The marketing story is also difficult because it involves increasing the awareness of ART diarrhea, which is under-recognized, and convincing physicians that Mytesi is needed as a solution when there are multiple prescription and OTC remedies. Mytesi has a unique profile of improving stool consistency without inhibiting peristalsis, but the company will need to shift the treatment algorithm before these details will be appreciated. Additionally, the company faces clinical risks with its development programs, similar to other companies performing clinical trials. The drug was approved for ART diarrhea on the basis of positive efficacy data, but has previously missed its endpoints for other indications such as irritable bowel syndrome, so there is no guarantee that positive data will translate between indications. The drug has been in development since the 1990s and the first patents expire in 2022, although there currently is not a pathway to develop generics to drugs approved under the FDA’s botanical guidance. And finally, the company faces significant dilution risk in its efforts to reduce its debt load and to finance future operations, and it may be forced to reduce spending or seek alternate financing.
Company description: Advancing diarrhea treatment
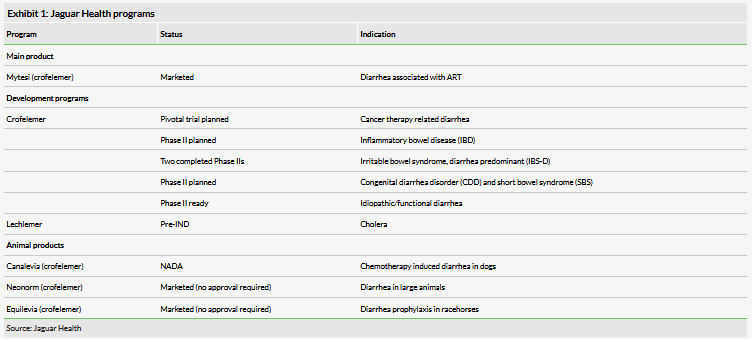
Jaguar Health is a pharmaceutical company focused on the marketing and development of crofelemer, a drug for the treatment of diarrhea in various patient populations. The product is currently approved and marketed in the US under the trade name Mytesi for the symptomatic relief of non-infectious diarrhea in adults with HIV/AIDS on anti-retroviral therapy (ART). The company has planned clinical programs to expand Mytesi use into a range of other possible diarrhea indications (Exhibit 1). Additionally, the company is developing the same drug for animal use under the brand Canalevia for dogs and currently markets it as Neonorm or Equilevia for large animals, although these programs are significantly deprioritized in favor of advancing Mytesi. Finally, the company has identified a similar molecule (lechlemer), which it is investigating for the treatment of cholera in preclinical studies. This program if it advances could qualify the company for a priority review voucher for targeting an underserved tropical disease.
Crofelemer background and mechanism
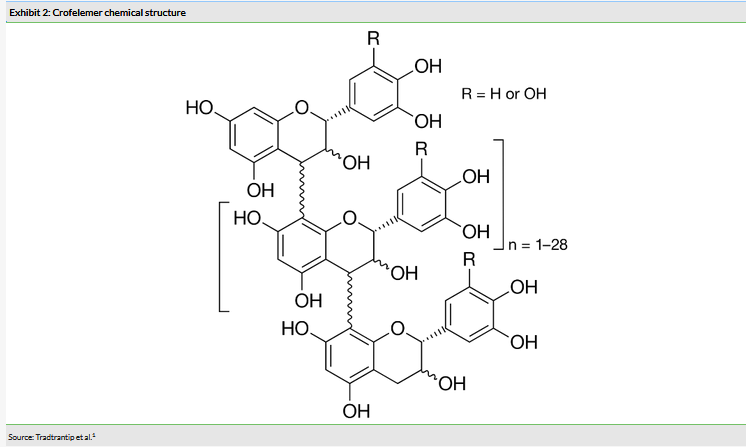
Crofelemer is a drug isolated from the latex of the Croton lechleri tree in the Amazon (NASDAQ:AMZN), the dragon blood plant. It is only one of two prescription drugs approved by the FDA under the botanical drug guidance, which allows for the approval of products from a botanical source where the product is not a pure substance. The active component of crofelemer is a mixed proanthocyanidin oligomer of indeterminate chemical structure (Exhibit 2)1 that is administered orally.
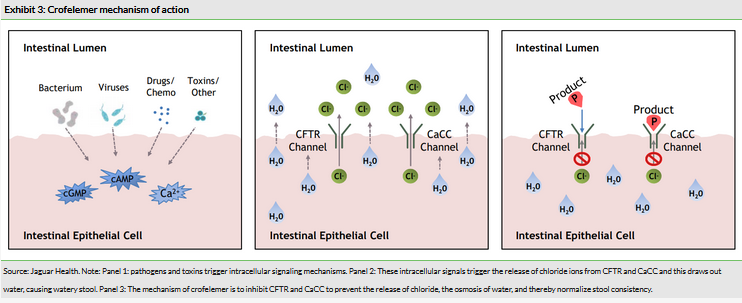
Diarrhea is typically triggered by exposure of cells of the intestine (enterocytes) to pathogenic bacteria or virus, the toxins these species produce, or to other chemical toxins such as drugs. These triggers lead to a signaling cascade in the enterocytes that ultimately culminates in the activation of the CFTR and the calcium dependent chloride channel (CaCC). Both of these proteins are transmembrane ion channels responsible for regulating the concentration of chloride ions in the lumen of the gut. By extension, these channels regulate the concentration of water in the gut as the chloride and other ions drive water into the gut via osmosis. The increased fluid improves motility and ultimately the pathogens or toxins are eliminated from the gut.
Crofelemer is an inhibitor of both CFTR and CaCC, and therefore by inhibition of these proteins can prevent the formation of watery stool. CFTR, as the name implies, is also present in the lungs and other organs and is implicated in the pathogenesis of cystic fibrosis in an unrelated fashion (due to a loss of function mutation), but there is no threat of off-target effects of crofelemer in other organs because it is not systemically absorbed. These proteins have been directly implicated in the pathogenesis of both a range of infectious diarrhea conditions2 as well as drug induced diarrhea3 and inhibition is effective at preventing watery stool in animal models.
History of crofelemer
Crofelemer as a therapeutic agent has a long business history. The drug was initially discovered at Shaman Pharmaceuticals in the 1990s, a company headed at the time by current Jaguar CEO Lisa Conte. Shaman was also developing crofelemer for the treatment of ART associated diarrhea, but declared bankruptcy in 2001 after the FDA requested additional clinical trials. The intellectual property was then purchased by Napo Pharmaceuticals (also headed by Lisa Conte) with the goal of pursuing the requested clinical studies with the support of development partners. The company unsuccessfully attempted to develop the drug for irritable bowel syndrome with diarrhea (IBS-D) with partner Trine Pharmaceuticals after a Phase IIb study failed to show improvement in stool consistency (although it reached statistical significance for improvement in pain). Napo then shifted its focus back to ART associated diarrhea and partnered with Salix Pharmaceuticals in 2008 to finance the completion of a Phase III study in exchange for rights to US and European markets. The drug was approved in 2013, under the trade name Fulyzaq. However, Napo sued Salix for breach of contract and accused the company of deliberately deprioritizing crofelemer in favor of Salix’s own drug Xifaxan, which had popular use off label as a treatment for IBS-D (Xifaxan has subsequently been approved for IBS-D). In the meantime, in 2013 the initial incarnation of Jaguar was formed as an opportunity to develop crofelemer for animal use, the rights to which were uncontested. Also during this period, Salix was embroiled in a scandal involving channel stuffing and the company was acquired by Valeant Pharmaceuticals (NYSE:BHC) (now Bausch Health). The lawsuit was settled in 2016 and all rights to crofelemer transferred back to Napo, which began selling the drug again in 2017. Napo was subsequently acquired by Jaguar in 2017, forming the current incarnation of the company.
Crofelemer is protected by three Orange Book patents covering the use of the drug for the treatment of secretory diarrhea broadly (expires in 2022) and ART associated diarrhea (two patents, expiring in 2031). All patents are on the method of use because as a natural substance, crofelemer is not subject to composition of matter patents. We would expect these 2031 patents to be challenged if the drug is successful. However, the product’s special status as a botanical extract may give it additional IP protection because there is currently no established pathway for the development of generic versions of drugs approved under the botanical drug guidance of the FDA. The agency has stated that it intends to provide a product specific guidance document on crofelemer to aid in the development of such generics, although the document has not been published, and there is no timeline provided. This is in line with greater efforts of the agency to promote the approval of generic drugs, and we do not consider the special status of Mytesi to be perpetually protective.
Clinical trial experience
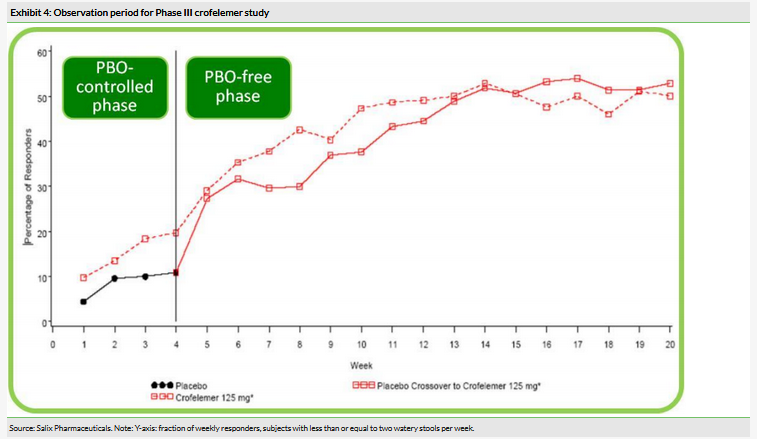
Crofelemer has been examined in a range of clinical studies against diarrhea indications. For its approved indication of ART associated diarrhea, it was evaluated in a 374-person, placebo-controlled Phase III study.4 The trial examined three dose levels of the drug (125mg, 250mg and 500mg bid) versus placebo, and the selected dose was examined in a one-month evaluation phase, followed up by a five-month uncontrolled observation period. The primary endpoint of the study was the number of ‘monthly responders’: subjects with less than or equal to two watery stools per week for more than two weeks out of a month. It was found that the selected dose (125mg bid) had a monthly responder rate of 17.6% compared to 8.0% for the placebo (p=0.0096) at the end of the one-month evaluation period. However, patient responses continued to improve over the five-month evaluation period (Exhibit 4).
The rate of adverse events in the study was lower for the crofelemer arm than for placebo. A larger analysis provided on the FDA label for Mytesi looking at all the patients across trials that received 125mg of drug for ART associated diarrhea compared to placebo (504 patients total) found a relatively benign safety profile. The most common adverse events that occurred more frequently than placebo were upper respiratory infection (6%), bronchitis (4%) and cough (4%).
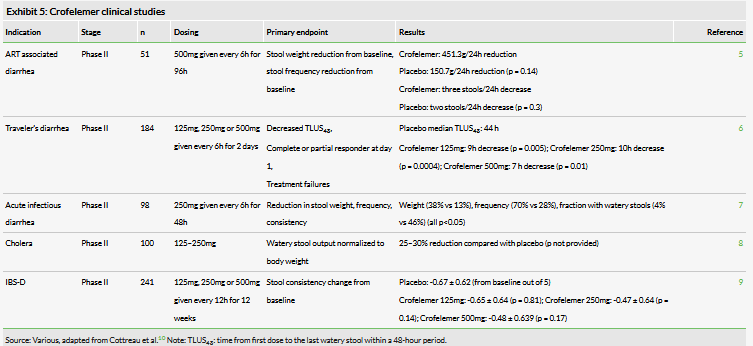
The results from other clinical studies have been mixed (Exhibit 5). The drug previously showed a positive treatment effect for traveler’s diarrhea, as measured by a reduction in time to the first solid stool (TLUS48). Patients reported solid stool in seven to 10 hours on crofelemer, compared to 44 hours on average with placebo. In a separate study, the drug also demonstrated improvement in a range of metrics for patients with acute infectious diarrhea: improvement in weight, frequency, and consistency of stools, as well as a greater number of resolutions within the first 48 hours (79% vs 28% p
However, in other trials, even though positive trends were observed, these effects did not reach statistical significance. Of particular importance is the IBS-D clinical study performed in collaboration with Trine Pharmaceuticals in patients with IBS-D. The study failed to demonstrate a statistically significant improvement in stool consistency. However, the study’s primary investigators noted that there appeared to be improvement in the number of discomfort-free days for patients in the study: after three months on the highest dose (500mg bid), 24.3% of patient days were discomfort free with crofelemer compared to 13.1% without (p=0.03).
The HIV associated diarrhea market
Mytesi is approved in the US for the treatment of non-infectious diarrhea in HIV patients on ART (HIV-D) and is the only drug approved with this indication. According to the US Department of Health, there are approximately 1.1 million people living with HIV in the US, of which the majority (six out of seven) are diagnosed. The department estimates that an additional 38,700 became infected in 2017. However, despite the widespread availability of ART treatments to control the disease, there remains room to improve adherence: a 2011 meta-analysis found an adherence rate of 62%.11
Diarrhea is common in patients with HIV, although results vary: one survey suggested that approximately 60% of patients receiving ART had diarrhea in the preceding month.12 There are multiple causes of the condition in these patients that can be roughly broken into two classes: infectious and non-infectious diarrhea. Infectious diarrhea is when an opportunistic infection colonizes and irritates the gut, whereas non-infectious diarrhea can either be caused by ART treatment (ART treatment associated diarrhea) or ‘HIV enteropathy’ in which the HIV infection itself leads to deterioration of the lining of the gut.
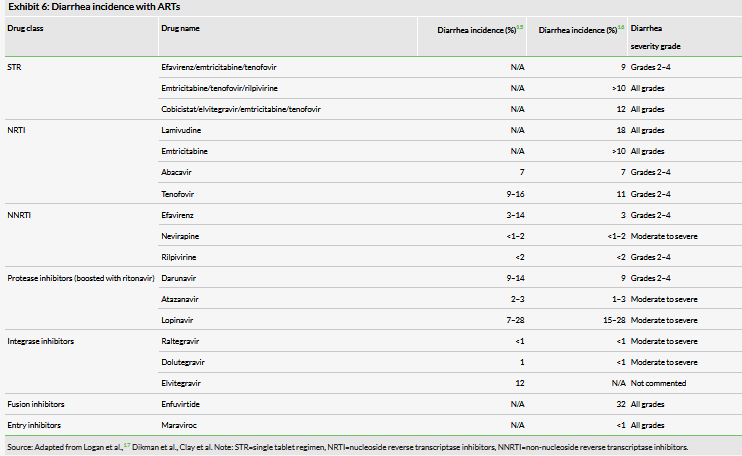
Clinical trial data suggests that up to 19% of diarrhea cases are attributable to ART therapy.13 One study chronicling the advent of ART therapy from 1995 to 1997 found that incidence of diarrhea remained constant in HIV patients, but the infectious variety declined from 53% of cases to 13% of cases as ART became more established.14 This reduction in infectious diarrhea can cause diarrhea as a whole to be under-recognized as a result of ART, because diarrhea rates may not change significantly when patients begin receiving ART, but these patients may see improvement when treated with Mytesi.
The HIV treatment market is constantly evolving as new anti-retroviral agents are developed and regimens are refined. Although improvements have been made, diarrhea continues to be a significant medical need in this patient population. Jaguar studied this in a retrospective study using NIH data from 38 clinical trials and found that diarrhea rates in these patients have fallen insignificantly between 2008 and 2016 and are approximately 18%. An example of this phenomenon is the case of the nucleotide reverse transcriptase inhibitor (NTRI) tenofovir, which is the cornerstone of most ART regimens. Tenofovir has been reported to be associated with diarrhea rates as high as 16%, depending on the study. It is provided as one of two commonly available prodrugs: tenofovir disoproxil fumarate (TDF, Truvada) is the historically available molecule, but tenofovir alafenamide (TAF, Vemlidy) was subsequently developed as an alternative that allows for substantially lower circulating levels of drug and the potential to avoid the associated nephrotoxicity. Both prodrugs are currently in use, but there is a trend toward increasing use of TAF containing regimens. However, when considering rates of ART associated diarrhea, TAF does not appear to provide a benefit and had similar rates of associated diarrhea in clinical trials (5% for both TAF and TDF, per the Vemlidy label).
In addition to ART associated diarrhea, Mytesi can potentially be used to treat diarrhea stemming from HIV enteropathy. HIV enteropathy is a negative diagnosis characterized by the presence of diarrhea without any infectious or other identifiable cause. The pathology of HIV contributes to a progressive deterioration of the lining of the gut that culminates in chronic diarrhea after many years of treatment. Unfortunately, there is little epidemiological data to separate HIV enteropathy from other forms of HIV associated diarrhea.
Commercial hurdles
Jaguar faces a unique set of commercial hurdles marketing Mytesi. First, the company must communicate the importance of treating diarrhea in this patient population. The extent of diarrhea in HIV infected individuals is underappreciated, as is the degree to which it affects both their quality of life and adherence to their ART regimen. ART has had little impact on rates of diarrhea, but this is largely due to high baseline rates, as described above. Additionally, although treatment has improved, ART has historically been associated with a range of more serious adverse effects than diarrhea, and therefore the condition has not had top priority. The company therefore engaged in efforts to communicate both the quality of life impact that treating HIV-D can have, as well as the potential impact of treatment on adherence of ART regimens. The company collected data from its Phase III study that quantitatively demonstrates that treatment with crofelemer can improve a range of quality of life factors, in particular an increased ability to perform work and leisure activities (p
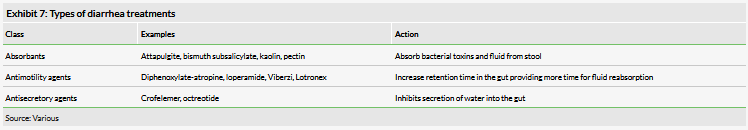
Additionally, the company must communicate the benefit of Mytesi over other commonly available diarrhea treatments. Antidiarrheals can be divided into three classes: absorbants, antimotility agents and antisecretory agents (Exhibit 7). Absorbants bind bacterial toxins and water to improve stool consistency, but are rarely used for HIV-D because of the potential to interfere with oral ART medications. The antimotility agents are the most widely employed for diarrhea by slowing the transit of stool. However, these agents can cause constipation as a side effect, especially in cases where the diarrhea may be intermittent, and may not be appropriate for use as a prophylactic in these cases. Moreover, antimotility agents can be contraindicated for certain infectious forms of diarrhea. Antisecretory agents, such as Mytesi, operate by decreasing the amount of water delivered to the gut. Octreotide is the only other antisecretory agent approved in the US, although it is used off label for this purpose. Results are inconclusive for the treatment HIV-D, and its use is limited due to side effects.
Jaguar currently has nine salespeople promoting the drug in the US following a recent salesforce reduction of up to 10 that was announced in August 2019. In order to expand its commercial footprint and reach more providers, Jaguar signed an agreement in June 2018 with RedHill Biopharma to co-promote Mytesi in 36 territories across the US (details on which specific territories or areas have not been disclosed) that are not currently being served by Jaguar’s internal salesforce. Sales under the agreement have been insignificant to date. Further details have not been released. Additionally, in September 2018, the company signed an agreement with Knight Therapeutics to commercialize the drug in Canada and Israel. Jaguar is entitled to a transfer price as well as up to $18m in regulatory and sales milestones. The product has widespread reimbursement in the US: 90% of lives in AIDS Drug Assistance Programs (ADAPs), top 10 commercial insurers (245m lives), top 10 Medicare plans (2.4m lives) and Medicaid in all 50 states.
Cancer therapy related diarrhea
Although the specialty market of ART-related diarrhea in the US is of limited size, the commercialization of Mytesi in this space demonstrates Jaguar’s ability to operate a supply chain that started with sustainably harvested trees in the rainforest, and the effectiveness and safety of the product. Additionally, bringing Mytesi to market has required the company to develop refined messaging and educational initiatives regarding the benefits of a new way of treating diarrhea with a chronically safe agent, as opposed to the use of anti-peristaltic alternatives such as Imodium and Loperamide. The company has outlined a clinical pathway forward toward expanding the approved indications for crofelemer to leverage some of this groundwork.
The most advanced clinical program is cancer therapy related diarrhea (CTD), which is currently in ongoing investigator initiated trials (IIT). GI adverse events are common in patients being treated for cancer with estimates as high as 40% of standard dose chemotherapy patients.19 Diarrhea is also a common adverse event for a range of targeted cancer therapies. Notably, drugs that target the ErbB family of growth receptors, which includes EGFR and HER2, typically have diarrhea as the most common adverse event. This is true of both monoclonal antibodies targeting these receptors as well as a tyrosine kinase inhibitors (TKIs). Rates of diarrhea seen in clinical trials for this class range from around 45% for most to over 90% for Gilotrif (afatinib, Boehringer Ingelheim) and Nerlynx (neratinib, Puma, more detail below). Moreover, these can be complicating factors for treatment, and GI issues are a major cause of discontinuations, delays and dosage adjustments.
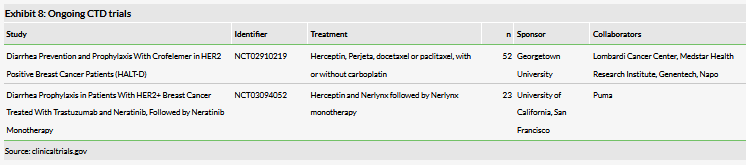
The ongoing clinical study is sponsored by Georgetown University and funded by Genentech (Exhibit 8). The study is examining the use crofelemer for diarrhea in patients undergoing treatment for HER2+ breast cancer. Diarrhea is a frequent problem in these patients, driven by both chemotherapy and the available targeted therapies. Herceptin (trastuzumab, $7.1bn 2018 sales) is the standard of care for these patients for both induction and adjuvant therapy and is associated with high rates of diarrhea in both settings. This clinical trial will be examining patients treated with Herceptin and Perjeta (pertuzumab, $2.8bn 2018 sales), the latter of which typically exacerbates patient diarrhea: 66.8% with Perjeta compared to 46.3% with Herceptin and docetaxel alone. The study is expected to have an interim readout on the first 23 patients in Q319, which we believe will inform the direction of the CTD program.
In addition to the above IIT, the product is being tested in another trial sponsored by the University of California San Francisco in collaboration with Puma Biotechnology, but without the participation of Jaguar. Patients in the study will receive Nerlynx as the adjuvant treatment. Nerlynx is a tyrosine kinase inhibitor (TKI) that was effective in clinical trials at preventing future metastases, but it is characterized by pervasive and severe diarrhea: 95% of patients have some form of diarrhea, 40% at grade 3. The Nerlynx label includes a direction to initiate prophylactic loperamide before the first dose. We expect this level of diarrhea to be a major limiting factor on sales of the drug ($200m in 2018), as well as potentially limiting future expansion of the drug into other indications.
Additionally the company recently reported top-line results from an animal study in dogs that is informative for CTD. The study examined 24 healthy beagles who were given the maximally tolerated dose of a specific undisclosed TKI and 125mg Mytesi twice a day (bid), four times a day (qid), or none at all (placebo). The study used a responder analysis similar to that used in the pivotal trial for HIV associated diarrhea, where responders were counted as dogs with less than seven watery stools per week for at least two weeks out of the 28-day treatment period. Seven of eight dogs in the qid group were responders (p=0.02) as were six of eight in the bid group (p=0.03) compared to three of eight in the placebo arm. Although the study is in dogs, these results are in line with previous studies and suggest that Mytesi may be useful in this particular variety of diarrhea.
The size of the addressable market for CTD will depend on the scope of future clinical study, and this will depend on future feedback from the FDA. The most attractive scenario would be one in which the drug can be tested and approved for diarrhea caused by a broad class of drugs (as opposed to individual cancer indications). We believe that this pathway should be possible considering the approval process for drugs used to treat chemotherapy induced nausea and vomiting (CINV). Drugs approved for CINV have been approved based on trials centering on groups of treatment regimens instead of certain diseases. One scenario would be if the drug can seek approval for diarrhea secondary to drugs that target the ErbB family. This would also expand the potential indications to other solid tumors such as lung cancer and renal cancer (among others). We estimate that there are over 100,000 person-years of drug exposure for these drugs per year.20 However, the most attractive scenario is one in which the drug would be broadly approved for alleviation of diarrhea secondary to both targeted treatment and chemotherapy. An estimated 650,000 patients in the US undergo chemotherapy each year,21 and the success of the CINV market highlights the opportunities. The leading CINV drug Emend (aprepitant, Merck) had peak US sales of $356m, with an estimated 149,000 patient-years per year.
The company met with the FDA in March 2019 to discuss the clinical trial design for the pivotal study, including discussions of a ‘basket’ study including multiple cancer agents. According to the most recent reports, the company is still finalizing the design with the agency for the anticipated basket label. However, the FDA did agree in the meeting that no additional preclinical safety studies or drug interaction studies were needed and that the product’s chemistry, manufacturing and controls (CMC) were sufficient.
Canalevia
The company is also advancing a different crofelemer product for a similar indication in dogs: Canalevia for chemotherapy induced diarrhea (CID). The company announced in June 2019 that it had completed the clinical data for the final technical section of its new animal drug application (NADA) and would be submitting it in Q319, and that these data indicate that the product is potentially also safe in puppies. We expect the primary market for this drug to be dogs undergoing chemotherapy for the treatment of canine lymphoma, which affects 20–100 dogs per 100,000.22 It is the primary cancer indication in dogs that is not treated surgically and can be well addressed with standard chemotherapies. Chemotherapy is also used post-surgically for soft tissue sarcomas (35–122 per 100,000), which may provide an additional market.23
The company has also stated that it intends to file for approval of the product for exercise-induced diarrhea (EID) in dogs. The company completed safety studies for the program in 2015. The indication is poorly understood and there is limited research into the subject, but it appears that strenuous exercise can lead to immune disturbances that translate into gastrointestinal irritation in some dogs. This has been studied the most extensively in Alaskan sled dogs, where it was seen that dogs had increased frequency of gastric erosions and ulcerations as well as increased intestinal permeability following a race.24 We expect this indication to be minor compared to CID given its poorly defined nature and intermittent presentation.
The company is seeking approval through the Minor Use and Minor Species Act (MUMS), intended to promote approval of animal drugs that are used in either limited indications or in less-prevalent species. It allows drugs to be marketed before all the necessary efficacy data has been collected and allows for post-marketing studies to provide this information. The program is modelled on the orphan drug program for human indications, and its structure is fundamentally similar. Canalevia has already received a MUMS designation for CID and the FDA has indicated EID would qualify as a minor use. The company has stated it intends to seek approval and launch the product for CID in 2020.
Other future programs
The company’s other human-use development programs are not currently in the clinic but it may expand into them depending on resources. All of these programs center on the anti-diarrheal activity of the drug. These include potential new indications such as short bowel syndrome (which has orphan drug designation) as well as revising previous programs such as IBS-D to build on prior clinical experience. The company has not released specific timing regarding the initiation of any of these proposed studies.
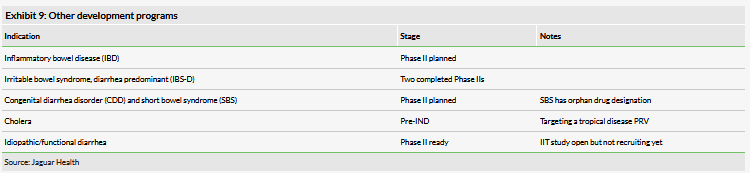
The company’s cholera program is different than for other indications because it is being performed with the drug candidate lechlemer instead of crofelemer. Lechlemer is also an extract of the Croton lechleri tree with similar properties to crofelemer and reportedly similar activity. Jaguar decided to use lechlemer instead of crofelemer because it will potentially enable it to receive a priority review voucher (PRV) if the drug is approved for the indication. PRVs are awarded to companies that receive approval for certain classes of disease that the FDA wants to encourage investment in. In this case, cholera is included in the tropical disease PRV program. Only newly approved drugs can receive a PRV, thus excluding crofelemer. After approval, the PRV can be sold to another drug company for a significant amount of money: previous sales have been for between $68m and $350m, and the latest transaction (to our knowledge) was for $105m (GW Pharmaceuticals to an undisclosed company).
Sensitivities
Jaguar faces a set of risks associated with both its commercial operations and future development. The company is commercializing Mytesi internally, which means it must bear the whole cost of its marketing, whereas a larger company could rely on a salesforce established to sell multiple drugs. The company has signed the promotional agreement with RedHill, although the latter is in a similar position as a small biotechnology company marketing a small portfolio of products and the collaboration has not produced any fruit to date. Adding to this is the fact that the marketing problem for Mytesi is particularly complicated because it involves changing certain perceptions with regards to the prevalence, severity and importance of addressing ART associated diarrhea. Campaigns with an educational component such as this can take many years to develop.
The company also faces the unavoidable risk associated with clinical development. The drug is in clinical trials for CTD, although these were not started by Jaguar and therefore may not be designed or executed with the intent of getting approval. Moreover, as with other investigator-initiated trials, the scope of these studies is more limited and the drug is only being tested in a small number of clinical sites. If the company choses to progress with the program, we expect pivotal studies to require a substantial number of patients (over 1,000) and carry significant costs.
Finally, the company must raise a substantial amount of capital to cover its marketing and development costs. We estimate that Jaguar will require at least $80m in additional capital to reach profitability in 2024 if the CTD program advances. If the CTD program does not advance, we forecast profitability in 2027 on ART sales alone and an additional expense of $12m. Raising capital to maintain operations could result in substantial dilution and addressing these needs in the past has diluted earlier shareholders: the company started 2019 with 0.9m split adjusted shares and has issued 10m more shares (or equivalents) and 19.5m warrants by August 2019.
The conversion of this debt is ongoing, and we expect further dilution to be very likely. Raising this much capital given the current market capitalization is unlikely to be feasible ($80m at $2.00 per share would result in 5x dilution) and if the company cannot either lift its share price substantially, obtain debt financing, or constrain its spending, future operations may be imperiled.
Valuation
We arrive at an initial valuation of $86.5m or $4.14 per diluted share based on a risk adjusted NPV analysis. We include Mytesi for ART and CTD in our valuation as well as Canalevia for canine CID.
The majority of our valuation is driven by the ART program, for which we forecast $74.1m in peak sales in 2031 at a penetration of 12%. We assume relatively low penetration given the availability of effective generic and OTC alternatives. We estimate an addressable market of approximately 70,000 individuals in the US who have HIV, are diagnosed, adherent and have non-infectious diarrhea. We assume a conservative 2% price growth per year (from the current $668 per month), and a 20% gross-to-net sales discount. COGS for Mytesi have historically been high at approximately 50%, although we see this improving progressively with volume to 25%. We also assume $6m in S&M overhead and 10% marketing expenses. Our model also includes revenue from the Knight agreement to market in Canada and Israel. We assume a transfer price of 50% of COGS and $13m (of a possible $18m) in milestones. We assume Knight will begin commercialization in these geographies in 2021. Our discount rate is 10%, which is our standard for approved products.
Of the company’s proposed development programs, we include CTD because it is the only program with ongoing clinical studies, albeit studies not initiated by the company. If the ongoing IIT studies are positive, we expect Jaguar to initiate pivotal studies in 2020. We expect a pivotal trial for CTD approval to be large with over 1,100 patients (based on previous approvals for CINV), but this is mitigated by a low per-patient cost of $10,000 as we expect timelines to be quick as in previous studies. Our model assumes the drug will be approved broadly for CTD, including patients on chemotherapy (as opposed to exclusively patients receiving TKIs or other ErbB inhibitors). We model approval in 2022 with similar pricing, COGS, etc, to the ART diarrhea program. We model lower S&M overhead for this program ($5m) as we expect there to be fewer headwinds convincing physicians of the importance of addressing this indication. Our probability of success for this program is 30%, which is our standard probability for programs in Phase II. We may also adjust this parameter based on the indication targeted for pivotal trials. Our discount rate is 12.5%, which is our standard for products in development.
We also include Canalevia for canine CID with lymphoma and STS as initial indications. We model pricing of $67 per month given the increased pricing pressure in the cash-pay veterinary market. Our probability of success for this program is 60% based on the stage and would be higher if we could evaluate the efficacy data being provided to the agency as part of the NADA.
Each of these indications is modelled to 2031, when the product’s Orange Book patents expire. Although there is not a pathway to develop generics for botanical drugs such as crofelemer, given the agency’s focus on removing barriers to generic drug development, we expect it to facilitate market entrants in that event. We also only model Jaguar marketing these products in the US as we believe anything else is beyond the company’s capacity short of partnerships such as those with Knight.
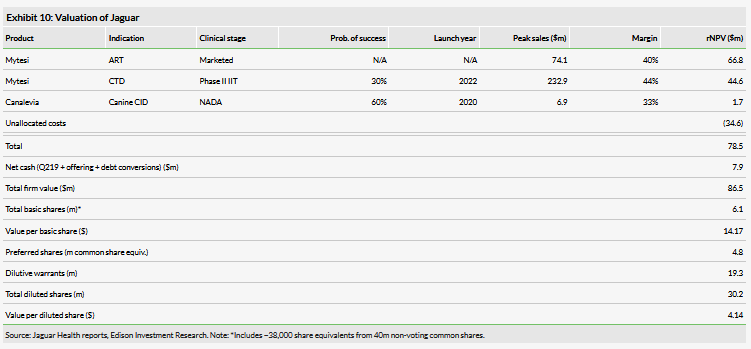
We also include a line item for unallocated costs to offset cash flow associated with corporate overhead and other factors. This negative NPV of $34.6m is driven primarily by high G&A expenses of approximately $12m per year. We may adjust this in the future if the company institutes cost controlling measures.
Financials
The company recently underwent a major effort to recapitalize and eliminate a portion of its debt. This process took approximately six months to complete following the end of 2018 and involved multiple elements:
The process has resulted in substantial dilution to the company’s shares. It ended 2018 with the equivalent of 0.9m split adjusted shares, which has increased to 10.9m common shares or equivalent in convertible preferred shares as of August 2019. Moreover, the company has issued 19.2m warrants so dilution will likely continue. Including these dilutive securities, this corresponds to a dilution of 97% in the past eight months. In addition to the above bridge notes, the company ended the quarter with $7.8m in notes (10% maturing December 2020), of which $1.1m has subsequently been converted to stock under exchange agreements. The elimination of this remaining debt may result in further dilution.
We expect the company to require substantial additional capital to deliver on its development plans. We forecast that the company will need no less than $80m in additional capital to deliver on its current development plants and reach profitability in 2024. We include this as debt for illustrative purposes in our forecasts: $30m each in 2020 and 2021, and $20m in 2022. However, if the company finances these costs through equity, we would expect substantial further dilution.
The company reported revenue of $1.7m for Q219, roughly flat on a sequential basis ($1.6m Q119), but almost doubled (up 92%) year-on-year. The revenue is almost entirely attributable to Mytesi, with a small portion from sales of its Neonorm products ($21,000). Operational expenses were similar to previous periods and dominated by G&A: $3.2m versus $1.7m for R&D and $2.2m for S&M. The loss reported for the period was $16.7m, which is not necessarily representative as it includes $6.5m in non-cash charges associated with the company’s refinancing and a $4m write-down on its intangible assets (for undisclosed reasons). We expect the reported loss in 2020 to be lower ($28.5m) due to fewer non-cash charges, but for there to be $22.9m negative cash flow for the year.