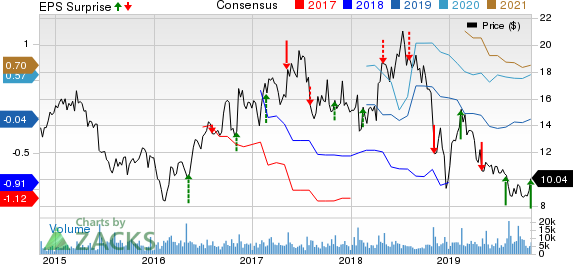
Ironwood Pharmaceuticals, Inc. (NASDAQ:IRWD) reported third-quarter 2019 adjusted earnings of 40 cents per share, surpassing the Zacks Consensus Estimate of 10 cents. In the year-ago period, the company had reported a loss of 38 cents.
The company turned profitable for the first time in the second quarter of 2019, following the separation of its Soluble Guanylate Cyclase (sGC) pipeline for treating serious and orphan diseases into a new public entity, Cyclerion Therapeutics, Inc. (NASDAQ:CYCN) in April 2019.
Total revenues of $131.2 million comprehensively beat the Zacks Consensus Estimate of $96.01 million. Revenues nearly doubled year over year. The significant increase in revenues was due to certain negative adjustment related to Linzess sales in the year-ago quarter and one-time payments made during the third quarter of 2019 related to amendments of license/collaboration agreements for Linzess.
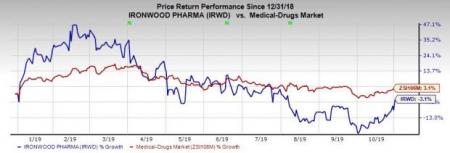
Ironwood’s shares rallied almost 6.5% on Oct 31, following the impressive results. However, shares of Ironwood have decreased 3.1% so far this year against the industry’s increase of 3.1%.
Quarter in Detail
As reported by partner Allergan plc (NYSE:AGN) , Ironwood’s key marketed product — Linzess —generated net sales of $214.7 million in the United States, up 4.8% year over year. Ironwood and Allergan equally share Linzess’ brand collaboration profits or losses.
Ironwood's share of net profits from sales of Linzess in the United States (included in collaborative revenues) was $84.6 million in the third quarter, up approximately 61.8% year over year. Net commercial profit in the reported quarter was $134.4 million.
Per data provided by IQVIA, volume of prescribed Linzess capsules in the third quarter increased about 15% year over year.The company stated on its earnngs call thatLinzess became the most prescribed drug in its approved indications during the third quarter.
Sales of linaclotide API to Ironwood’s Japanese partner, Astellas Pharma, was $0.6 million compared to the $10.3 million in the year-ago period.
2019 Guidance Raised
Ironwood increased its guidance for total revenues to the range of $410-$420 million from the previously announced range of $370-$390 million for 2019 on the back of strong growth momentum in Linzess sales. Net interest expenses are anticipated to be approximately $35 million.
The company also raised its EBITDA guidance for 2019 including the impact of the business separation. The company expects adjusted EBITDA to be more than $130 million (previously $65 million), reflecting strong top-line growth, and license and milestone payments recorded during the third quarter. It also expects net sales of Linzess to grow by mid-single digit percentage point (previously low- to mid-single digit).
The company expects to save $25 million over the next five years by shifting its headquarters to downtown Boston from its current location in Cambridge, MA. The relocation was completed in October.
Pipeline Updates
Linzess is approved in the United States for the treatment of adults with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation. Ironwood and Allergan are looking to expand Linzess’ label into additional symptoms and develop the drug as a non-opioid, pain-relieving agent for IBS patients.
In June, Ironwood and Allergan announced positive top-line data from the phase IIIb study on Linzess for treating multiple abdominal symptoms in adult patients with IBS-C.
In May, the companies initiated a phase IIb study for evaluating three once-daily doses — 300, 600 and 1,200 mcg — of MD-7246 (delayed release formulation of Linzess) to treat abdominal pain associated with IBS with diarrhea. The companies are enrolling patients in the study and top-line data is expected by mid-2020.
Ironwood is also developing an interesting candidate called IW-3718. It is currently enrolling patients in two identical phase III studies, evaluating IW-3718 for treating gastroesophageal reflux disease. Results from both studies are expected in the second half of 2020.
Global Collaborations and Partnerships
Ironwood has a license agreement with Japan based, Astellas Pharma. Ironwood supplies linaclotide API to Astellas Pharma for manufacturing and developing Linzess for sale in Japan. Ironwood amended this license agreement with Astellas Pharma for an upfront payment of $10 million and recorded it as revenues in the third quarter. Per the amended terms, Astellas will be manufacturing linaclotide API in Japan and Ironwood will receive royalties on net sales of Linzess in the country.
In China, Hong Kong and Macau, Ironwood has a collaboration agreement with AstraZeneca (NYSE:AZN) for development and commercialization of Linzess. Ironwood also amended the agreement in the third quarter. Per the amended terms, Ironwood has granted AstraZeneca exclusive rights to develop, manufacture, and commercialize Linzess in these territories. AstraZeneca will be responsible for all expenses associated with Linzess and Ironwood will no longer be jointly funding the development and commercialization of Linzess neither will it share profits on the drug’s sales in China. The company will receive royalties on net sales of Linzess in China and included territories.
Per the terms of the amended agreement, Ironwood is eligible to receive payments up to a total of $125 million, which includes $35 million in non-contingent payments and $90 million payment contingent to achieving certain annual net sales targets.It received $32.4 million of the non-contingent payments and recorded as revenues in the third quarter.
In January, Linzess was granted marketing approval by Chinese regulatory authorities to treat adults with IBS-C. AstraZeneca plans to launch the drug in China for IBS-C soon.The drug is already available and the field force has completed training.
Our Take
Ironwood reported encouraging third-quarter results with sales and earnings beating estimates. Linzess’ prospects look encouraging owing to strong demand trends and the drug’s expansion to new patient population and geographies. Moreover, amendments to collaboration agreement in China will likely lead to decline in operating expense going forward.
The new Ironwood entity has solid potential and its focus on the gastrointestinal product of the commercial portfolio and pipeline is impressive.
The company’s $139.2 million cash resources as of September-end, separation of sGC pipeline and a strong partner in Allergan bode well.
Zacks Rank
Ironwood currently sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +24.5% per year. So be sure to give these hand-picked 7 your immediate attention.
Allergan plc (AGN): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Ironwood Pharmaceuticals, Inc. (IRWD): Free Stock Analysis Report
Cyclerion Therapeutics, Inc. (CYCN): Get Free Report
Original post
Zacks Investment Research