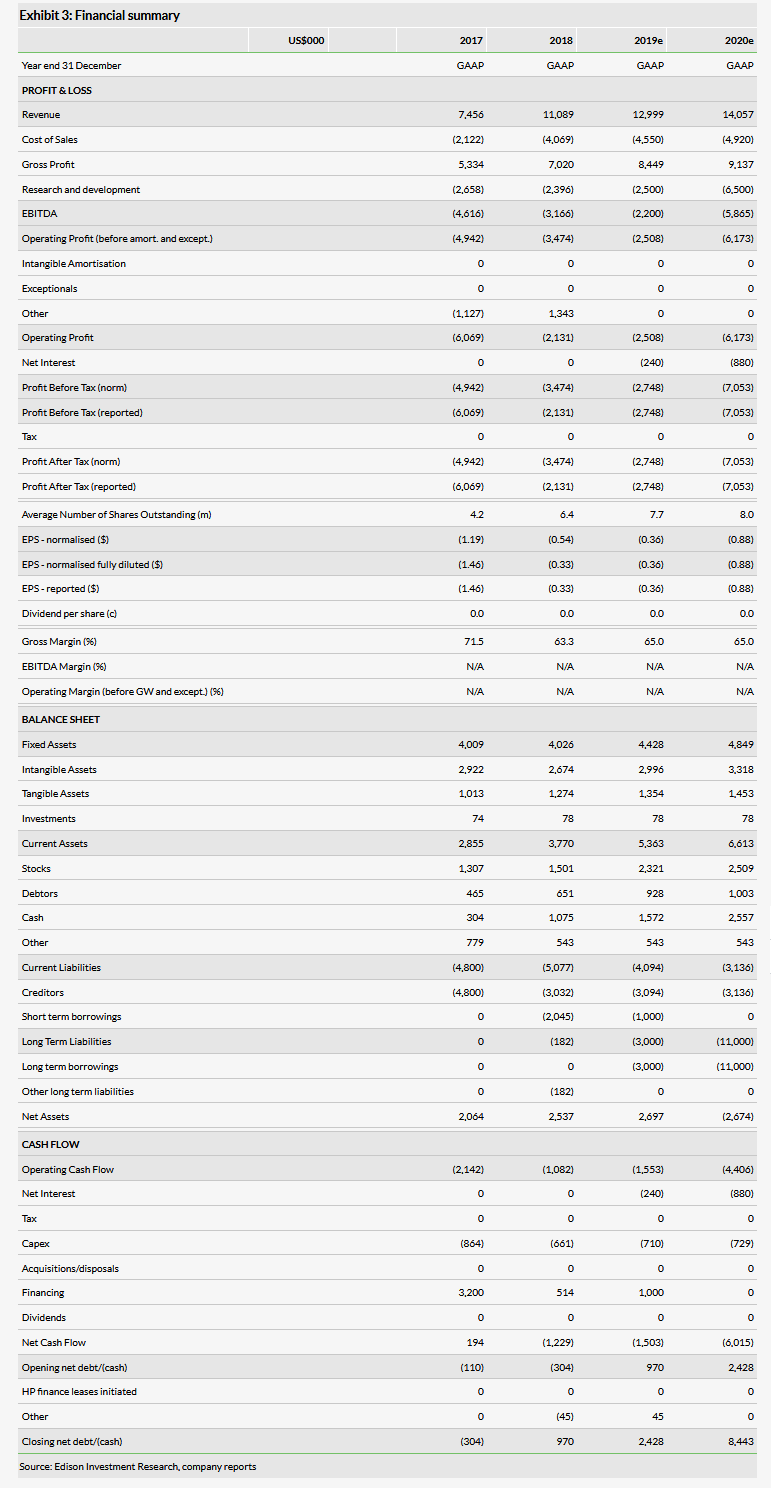
International Stem Cell Corp (OTC:ISCO) reported strong FY18 revenues of $11.1m, up 48.7% compared with 2017 mainly due to the biomedical business, which had revenues of $9.3m, up 78.4% year-on-year. The profitability of the biomedical business continues to improve as well, with operating income of $3.3m, up 78.2% compared with the previous year. The company has also reported that the 12-patient Phase I trial of ISC-hpNSC in Parkinson’s disease (PD) is now fully enrolled with complete data expected in H120.
Strong sales
ISCO’s commercial operations leverage its human parthenogenetic stem cell (hpSC) technology and generate revenues to partially offset R&D spending for therapeutic development. Lifeline Skin Care (LSC) develops and sells skincare products and Lifeline Cell Technology (LCT) produces human cell culture products for testing. Together they generated $11.1m in sales in 2018, up 48.7% compared with last year and provided $2.4m in operating profit which was used to fund R&D.
LCT, the engine of the commercial business
LCT develops, manufactures and commercializes over 130 human cell culture products, including frozen human “primary” cells and the reagents (called media) needed to grow, maintain and differentiate the cells. Cell types include endothelial, epithelial, fibroblasts, melanocytes, stem and smooth muscle among others.
The final cohort of PD trial completed
The third and fourth patients in the third cohort of four patients have been successfully dosed. As a reminder, patients in the study are being treated in three cohorts with 30m, 50m and 70m stem cells, delivered via intracranial injection. The single-arm, open-label study is being conducted at the Royal Melbourne Hospital in Australia. Clinical assessments are scheduled at six and 12 months following surgery with complete data expected in H120.
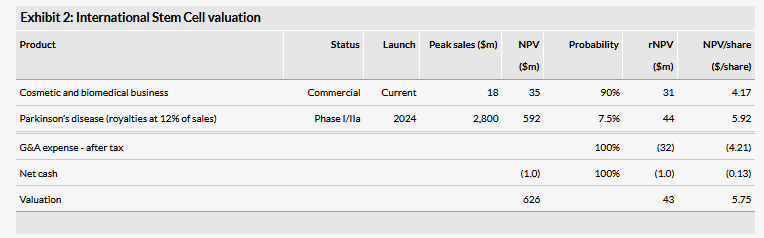
Valuation: $43m or $5.75 per share
We have adjusted our valuation to $43m or $5.75 per basic share from $42m or $6.16 per basic share. The increase in total valuation is mainly due to rolling forward our NPV while the per share valuation decreased due to a higher share count. We project that the company will need at least $40m in additional financing before profitability in 2024 (down from $45m due to higher revenues and continued cost controls), of which an additional $3m will be required by the end of 2019.
Business description
International Stem Cell is an early-stage biotechnology company developing therapeutic, biomedical and cosmeceutical applications for its proprietary stem form of pluripotent stem cells – human parthenogenetic stem cells. Its lead candidate is a cell therapy treatment for Parkinson’s disease.
Q418 results
The company reported strong FY18 revenues of $11.1m, up 48.7% compared with 2017 mainly due to the biomedical business, which had revenues of $9.3m, up 78.4% year-on-year. For the quarter, revenues were $2.2m, up 20.7% compared with Q417 mainly due to the biomedical business, which had quarterly revenues of $1.7m, up 43.4% compared with the same quarter last year. The cosmetics business, however, was down 19.7% for the year and now represents just 16.3% of total revenues compared with 30.3% in 2017. The operating profit of the commercial business increased 67.7% to $2.4m in 2018. For the company as a whole (including its therapeutics development programs), the operating loss was $3.5m for the year, down 29.7% from $4.9m in the previous year. We have made some adjustments to our model, decreasing our 2019 revenue estimate for the commercial business from $13.5m to $13.0m due to a slightly lower run rate than we modeled for the fourth quarter. We have kept SG&A roughly the same but reduced our 2019 R&D estimate to $2.5m from $6.5m due to continued cost controls at the company and the fact that the Phase I is now fully enrolled. We are also introducing 2020 estimates, including revenue of $14.1m, up 8.1% compared to our 2019 estimate.
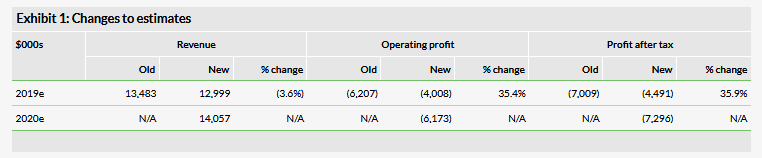
The company had $1.1m in cash on the balance sheet at the end of 2018. There was $2.0m in a related-party payable stemming from a promissory note that provided cash to the company from its co-chairman and CEO, with the note due and payable on 15 January 2019. Subsequent to the quarter end, the company entered into a conversion agreement to convert $1.0m of the note into 599,222 shares, with the remaining $1.0m now due on 15 January 2020. We project that the company will need at least $40m in additional financing before profitability in 2024 (down from $45m due to continued cost controls), of which an additional $3m will be required by the end of 2019.
Valuation
We have adjusted our valuation to $43m or $5.75 per basic share from $42m or $6.16 per basic share. The increase in valuation is mainly due to rolling forward our NPV while the per share valuation decreased due to a higher share count.
One key risk to remember stems from the capital structure, which potentially creates sizeable dilution risk for minority investors. To date, ISCO has relied primarily on funds from management in the form of a combination of convertible preferred shares, warrants and options to fund its growth so that on a fully diluted basis management controls a significant portion of the company. While management has not converted the bulk of its sizeable holdings investors need to consider the possibility of significant dilution risk at some point in the future. There remain approximately 14.4m potentially dilutive shares from 4.0m warrants, 4.4m options and 6.1m convertible preferred shares in addition to the 7.5m common shares outstanding. Also, investors should note that the convertible preferred shares are subject to anti-dilution provisions under certain circumstances, creating further dilution potential.