Intercept Pharmaceuticals, Inc. (NASDAQ:ICPT) gained 3.02% after the company posted mixed results for the first quarter. The company reported a wider-than-expected loss but beat on sales expectations.
The company incurred a loss of $3.03 per share in the first quarter, wider than the Zacks Consensus Estimate of a loss of $2.57 but narrower than the year-ago loss of $3.22.
Quarterly revenues were $52.2 million, up from $35.9 million in the year-ago quarter. Revenues also marginally surpassed the Zacks Consensus Estimate of $52 million.
Quarter in Detail
Ocaliva recorded $51.8 million of sales, down from $52.9 million recorded in the fourth quarter of 2018 but up from $35.2 million in the year-earlier quarter. Net sales in the United States came in at $38 million, while ex-U.S. Ocaliva net sales summed $13.8 million.
Ocaliva in combination with ursodeoxycholic (UDCA) was approved in the United States in 2016 for the treatment of primary biliary cholangitis (“PBC”) in adults with an inadequate response to UDCA or as monotherapy in adults, who are unable to endure UDCA. The drug was also granted a conditional approval by the European Commission. In February 2018, the drug’s label was updated in the United States to include a boxed warning and a dosing table that reinforced the existing dosing schedule in PBC patients with Child-Pugh Class B or C or decompensated cirrhosis.
Research and development expenses increased 19.9% year over year to $58.4 million, primarily due to more clinical development programs of Ocaliva for NASH. However, selling, general and administrative expenses increased 23.5% to $77.2 million.
2019 Outlook
For 2019, Ocaliva’s net sales are expected between $235 million and $245 million. Intercept continues to expect operating expenses of $470-$500 million for the year.
Pipeline Update
Obeticholic acid (OCA) is also being evaluated for other indications, including non-alcoholic steatohepatitis (“NASH”) and primary sclerosing cholangitis (“PSC”).
Earlier in 2019, Intercept announced positive top-line results from the pivotal phase III REGENERATE study of OCA in patients with liver fibrosis due to NASH. The company stated that the primary endpoint of the study — fibrosis improvement without worsening of NASH at 18 months — was achieved with the 25 mg daily dose of OCA.
Also, a numerically greater proportion of patients in both OCA treatment arms (receiving doses of 10 mg and 25 mg) met the primary endpoint of NASH resolution with no deterioration of liver fibrosis as compared to placebo. However, this did not reach statistical significance. Nevertheless, the study was required to attain one of the two primary goals per the FDA, which it did.
In April 2019, additional supportive REGENERATE data were presented during the Opening Ceremony of the International Liver Congress™ 2019, the 54th Annual Meeting of the European Association for the Study of the Liver (“EASL”). The data showed that OCA demonstrated robust efficacy across a range of additional histologic and biochemical parameters.
Intercept plans to file for an approval of OCA as a NASH treatment both in the United States and Europe in the third and fourth quarters, respectively.
The REVERSE study is designed to evaluate the efficacy and safety of Ocaliva in NASH patients suffering from compensated cirrhosis. The study is currently enrolling patients, which is expected to be complete by the end of the year.
Our Take
Intercept’s first-quarter results were mixed, as earnings lagged expectations but sales beat the same. Management’s efforts to increase awareness about the label update of Ocaliva in 2018 and expand sales force across the United States, thereafter, are reaping results. Solid growth in new patient enrollment is likely to drive sales in 2019.
Moreover, the favorable outcomes from the REGENERATE assessment are a big boost to Intercept. This news significantly buoyed investors’ sentiments, given the market potential of NASH and the fact that biotech bigwig Gilead Sciences, Inc. (NASDAQ:GILD) announced the failure of a late-stage study on selonsertib, involving patients afflicted with compensated cirrhosis (F4) due to NASH. This, in turn, puts Intercept ahead in the race to get a drug approved for NASH.
Pharma giant Merck (NYSE:MRK) , in January 2019, exercised its option to license NGM313, an investigational monoclonal antibody agonist of the β-Klotho/FGFR1c receptor complex that is currently being evaluated for the treatment of NASH and type II diabetes.
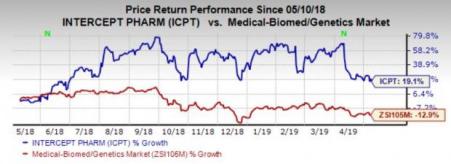
Shares of the company have rallied 19.1% in the past year compared with the industry’’s loss of 12.9%.
France-based Genfit’s (NASDAQ:GNFT) lead candidate elafibranor, which is in late-stage studies for NASH, also holds promise.
Zacks Rank
Intercept currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.v
This Could Be the Fastest Way to Grow Wealth in 2019
Research indicates one sector is poised to deliver a crop of the best-performing stocks you'll find anywhere in the market. Breaking news in this space frequently creates quick double- and triple-digit profit opportunities.
These companies are changing the world – and owning their stocks could transform your portfolio in 2019 and beyond. Recent trades from this sector have generated +98%, +119% and +164% gains in as little as 1 month.
Click here to see these breakthrough stocks now >>
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Genfit SA (GNFT): Get Free Report
Original post
Zacks Investment Research
