IntelGenx Corp. (OTC:IGXT) announced that it has resubmitted its new drug application (“NDA”) for Rizaport VersaFilm for the treatment of acute migraines to the FDA.
The company received a Complete Response Letter (“CRL”) from the FDA regarding the NDA in April 2019. The FDA cited the issues related to the Chemistry, Manufacturing and Controls section of the application. The agency requested additional information, but no new bioequivalence study was asked for.
We note that Rizaport is a patent-protected, proprietary, oral thin film formulation of rizatriptan benzoate, a 5-HT1 receptor agonist, and the active drug in Merck & Co.'s (NYSE:MRK) Maxalt. Rizaport offers a potentially advantageous therapeutic alternative for many migraine patients due to its convenient dosing, facile intake due to the lack of need for water, and neutral flavor. Rizaport is developed using VersaFilm, IntelGenx’s proprietary oral film technology.
Rizaport 5 mg and 10 mg were approved for marketing in Germany in October 2015 and in Luxembourg in April 2017 under the European Decentralized Procedure.
IntelGenx looks forward to working with partner, Gensco Pharma, to commercialize Rizaport in the United States upon FDA approval.
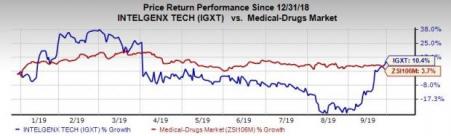
Shares of the company have gained 10.4% year to date compared with the industry’s growth of 3.7%.
We remind investors that, Allergan plc’s (NYSE:AGN) NDA for oral anti-calcitonin gene-related peptide (“CGRP”) candidate, ubrogepant is under review by the FDA for the acute treatment for migraine in adult patients. Allergan’s largest product, Botox, is approved for treating migraine and several other diseases. In 2018, three anti-CGRP drugs received approval from the FDA for treating migraine — Amgen/Novartis’ Aimovig, Eli Lilly’s (NYSE:LLY) Emgality and Teva Pharma’s Ajovy.
IntelGenx currently carries a Zacks Rank #4 (Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98%, +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Allergan plc (AGN): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
IntelGenx Technologies Corp. (IGXT): Free Stock Analysis Report
Original post
Zacks Investment Research