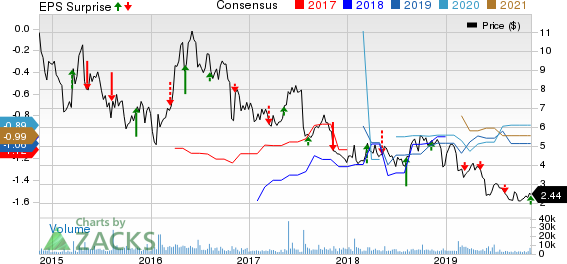
Inovio Pharmaceuticals, Inc.’s (NASDAQ:INO) adjusted loss of 27 cents per share in third-quarter 2019 was in line with the Zacks Consensus Estimate.
Adjusted loss in the quarter excludes the impact of change in fair value of derivative liability and loss on investments in affiliated entities. However, including this impact, Inovio’s loss of 25 cents in the third quarter narrowed from the prior-year loss of 27 cents.
Revenues in the reported quarter were $0.9 million, down from the year-earlier quarter’s $2 million. This year-over-year decrease was primarily due to lower reimbursement from AstraZeneca (NYSE:AZN) in relation to drug manufacturing activities. Sales also missed the Zacks Consensus Estimate of $3 million.
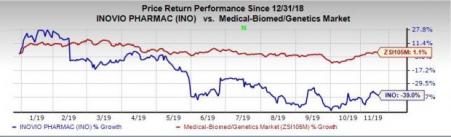
Shares of Inovio fell 2.5% in after-hours trading following the company’s earnings release on Nov 12. In fact, the stock has declined 39% so far this year versus the industry’s increase of 1.1%.
Quarter in Detail
Research and development expenses were $19.1 million in the third quarter, down 12.8% year over year, mainly due to lower employee compensation and drug manufacturing expenses.
General and administrative expenses decreased 16.2% to $5.7 million in the quarter owing to lower allocated depreciation expense and legal expenses.
Inovio had $93.8 million worth of cash, cash equivalents and short-term investments as of Sep 30, 2019 compared with $106 million on Jun 30, 2019.
Updates on VGX-3100 & Other Pipeline News
VGX-3100, an HPV immunotherapy, is the most advanced candidate in Inovio’s pipeline right now.
VGX-3100 is currently being evaluated in a phase III study (REVEAL 1) for the treatment of cervical dysplasia caused by papillomavirus (HPV). Top-line efficacy data from the REVEAL 1 program is expected by the fourth quarter of 2020. Another phase III study (REVEAL 2) is also on track as Inovio is recruiting patients in the United States and globally. On the conference call, management stated that data from both REVEAL studies will support a biologics license application (BLA) for VGX-3100.
In July, Inovio completed enrollment in a phase II study evaluating VGX-3100 for the treatment of anal dysplasia caused by HPV. In the same month, it concluded enrollment in another phase II study on VGX-3100 for vulvar dysplasia caused by HPV. Inovio plans to report interim data from both studies at an upcoming medical conference in the first quarter of 2020.
This apart, Inovio is evaluating MEDI0457 (formerly INO-3112) in combination with AstraZeneca's anti-PD-L1 checkpoint inhibitor Imfinzi (durvalumab) for treating HPV-related cancers including the phase II study to treat head and neck cancer. AstraZeneca plans to complete this study by the third quarter of 2020. Inovio out-licensed MEDI0457 to MedImmune, a subsidiary of AstraZeneca.
Moreover, Inovio is developing a novel therapy, INO-3107, for treating patients with recurrent respiratory papillomatosis (RRP), an HPV-associated disease. The company plans to advance INO-3107as a rare orphan product within the first half of next year.
We remind investors that in July, Inovio announced a restructuring process to prioritize the development of its late-stage human papillomavirus (HPV) assets and reallocate capital to develop the fast-to-market product candidates. Following this, the company reduced its annual burn rate by 25% and cut workforce by 28%.
Inovio also decided to eliminate some early-stage R&D programs and terminated the phase I/II study on INO-5401 for the treatment of advanced bladder cancer.
Earlier this month, Inovio announced positive interim data from the phase II study on its immuno-oncology combo of INO-5401 and INO-9012 in combination with Regeneron (NASDAQ:REGN) and Sanofi’s (NASDAQ:SNY) PD-1 inhibitor Libtayo (cemiplimab) for addressing newly-diagnosed patients with glioblastoma (GBM).
Notably, in September 2019, Inovio received a $4.6-million grant from the National Institutes of Health to support the company's DNA-encoded monoclonal antibodies (dMAb) technology platform against antimicrobial-resistant infection.
This apart, the company is working on developing Ebola, Zika, Lassa Fever and Middle East respiratory syndrome (MERS) virus vaccines.
Inovio Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
Zacks Rank
Inovio currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Breakout Biotech Stocks with Triple-Digit Profit Potential
The biotech sector is projected to surge beyond $775 billion by 2024 as scientists develop treatments for thousands of diseases. They’re also finding ways to edit the human genome to literally erase our vulnerability to these diseases.
Zacks has just released Century of Biology: 7 Biotech Stocks to Buy Right Now to help investors profit from 7 stocks poised for outperformance. Our recent biotech recommendations have produced gains of +98%, +119% and +164% in as little as 1 month. The stocks in this report could perform even better.
See these 7 breakthrough stocks now>>
AstraZeneca PLC (AZN): Free Stock Analysis Report
Sanofi (PA:SASY) (SNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research