InMed Pharmaceuticals Inc (TSX:IN) recently reported results for FY19 and is on track for INM-755 for epidermolysis bullosa (EB) to be in the clinic by the end of 2019. The Phase I programme will consist of two trials, one in healthy volunteers with intact skin and the other in healthy volunteers with small wounds. A Phase I/II trial in EB patients is expected to begin in Q121. In glaucoma, the company has switched its lead candidate to INM-088, which has some specific advantages to the former lead, INM-085. Advanced preclinical and formulation development is expected to begin by the end of the year.
Entering the clinic by the end of the year
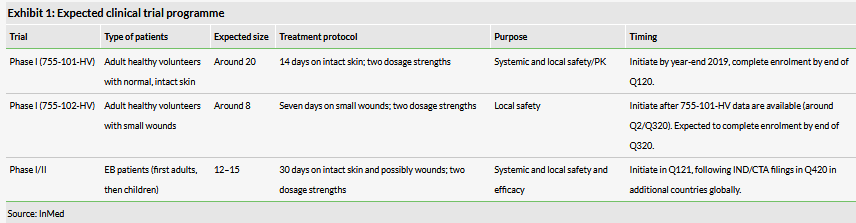
InMed is on track for initiating the Phase I programme for INM-755 in healthy volunteers by the end of 2019. There will be two separate trials that will be carried out serially as data from the trial in subjects with normal, intact skin are necessary for the trial in subjects with small wounds. We expect the Phase I programme to be complete in Q320 with the critical Phase I/II in EB patients starting in Q121.
Glaucoma coming into view
The glaucoma programme is advancing with a new lead candidate due to superior preclinical results. Advanced preclinical and formulation development is expected to begin by the end of the year with key IND-enabling studies expected to begin in H120 after discussions with regulators.
Biosynthesis progressing
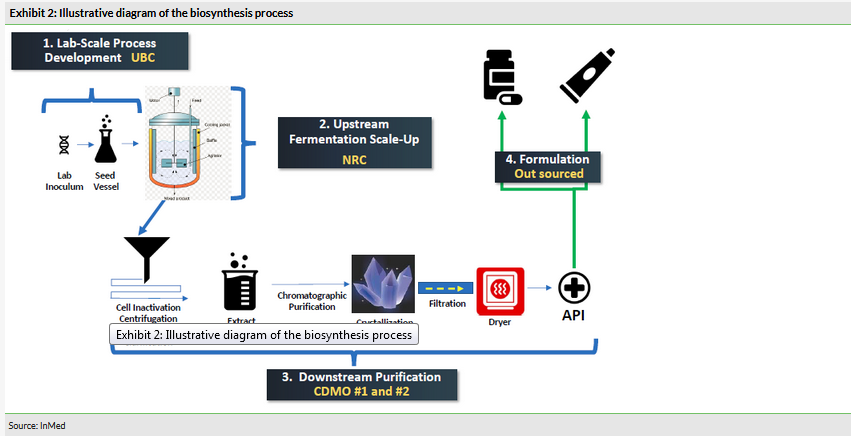
The company’s E. coli-based biosynthesis process continues to move forward. The company has optimised several fermentation parameters that are part of the Up Stream Process (USP) to maximise yield and initiated Down Stream Purification (DSP). InMed is also investigating an alternative process (the exact nature of which is undisclosed) that may have advantages in terms of cost and yield, and will decide which approach to move forward with in H120.
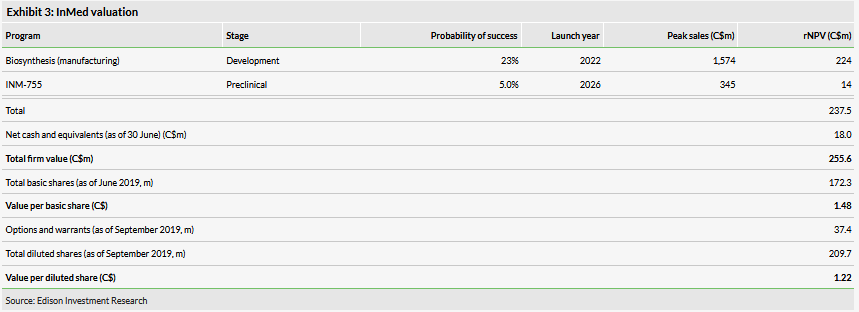
Valuation: C$256m or C$1.48 per basic share
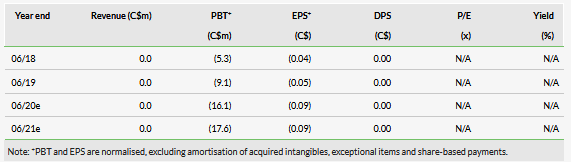
We have slightly adjusted our valuation from C$255m or C$1.48 per basic share (C$1.14 per diluted share) to C$256m or C$1.48 per basic share (C$1.22 per diluted share). The valuation increase of rolling forward our NPV was offset by pushing back our expectations for initial revenues from the biosynthesis business to FY22 from FY21 and lower net cash. InMed had C$18.0m in cash at 30 June and we believe this provides a runway into FY21. We now forecast the company will raise C$20m over the next two years to fund operations.
FY19 results update
InMed continues to be on track for initiation of the Phase I programme in healthy volunteers by the end of the year. The programme will consist of two separate trials (see Exhibit 1) that will be conducted in the Netherlands where there is less administrative burden to conduct a cannabinoid-based clinical trial. Trial 755-101-HV will consist of approximately 20 healthy volunteers with normal, intact skin and will evaluate the systemic and local safety, tolerability and PK of INM-755 cream. Trial 755-102-HV will have around eight healthy volunteers with small wounds to evaluate the local safety of the product, especially in people with broken skin (the small wounds will be created at the clinical site and will largely mimic the types of wounds typically seen in EB simplex patients). A Phase I/II in approximately 12–15 EB patients is expected to begin in Q121. Note that all these trials will be double blind and vehicle controlled. Importantly, the safety studies can be used as the basis for a clinical trial programme in other indications as INM-755 may have applications in other dermatologic indications involving inflammation, pain and itch.
The glaucoma programme is advancing. The company has chosen a new lead candidate (INM-088) due to superior preclinical results compared to the previous lead, INM-085, and its potential to treat other ocular disorders beyond glaucoma. Advanced preclinical and formulation development is expected to begin by the end of the year with key IND-enabling studies expected to begin in H120 following discussions with regulators. Exact timing for the initiation of human clinical studies is unclear but they could begin around H221.
Biosynthesis programme
With regards to the company’s E. coli-based biosynthesis programme, InMed has optimised several fermentation parameters that are part of the USP to maximise yield and has initiated the DSP.
The company is also exploring an alternative process (the exact nature of which is undisclosed) that may have advantages in terms of cost and yield (items of paramount importance in this industry). A decision on which process to move forward is expected by the end of the year. The selected process would then enter scale-up in H120 with GMP batch production expected in H220.
Valuation
We have slightly adjusted our valuation from C$255m or C$1.48 per basic share (C$1.14 per diluted share) to C$256m or C$1.48 per basic share (C$1.22 per diluted share). The valuation increase of rolling forward our NPV was offset by pushing back our expectations for initial revenues from the biosynthesis business to FY22 from FY21 (mainly for the sake of conservatism as FY22 begins in July 2021) and by lower net cash. The valuation per diluted share benefited from the fact that 13.9m options expired in July 2019 (an additional 16.6m warrants with a C$1.25 strike price expire in June 2020).
Financials
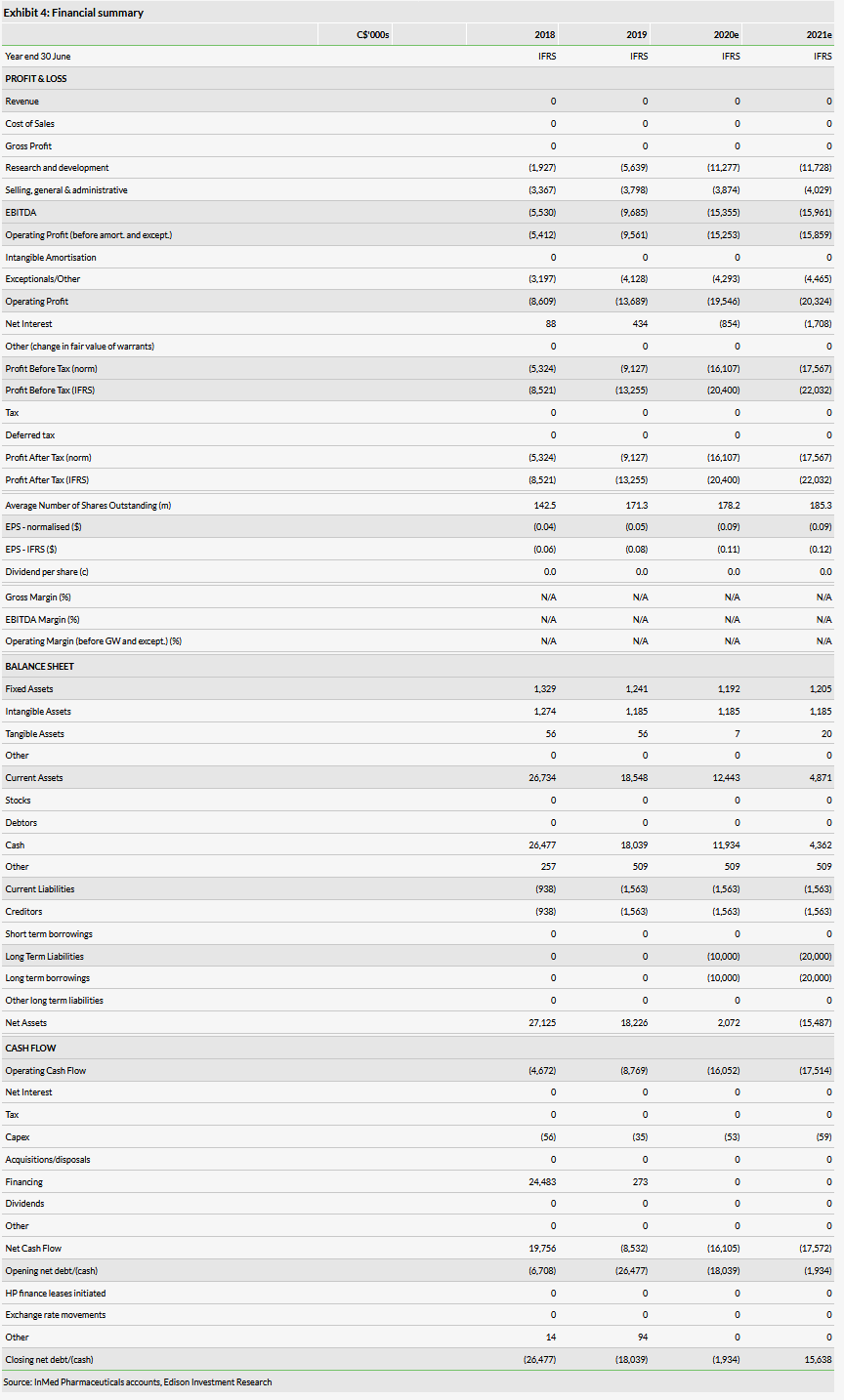
InMed reported an operating loss of C$9.6m in FY19 (the year ending 30 June 2019), up from C$5.4m in the prior year. R&D expenses were C$5.6m for the year, up from C$1.9m in FY18. We have increased our R&D estimates by C$1.3m in FY20 to C$11.3m as spending was higher than we initially expected in Q4. We also introduce FY21 estimates, which include C$11.7m in R&D spending and C$15.9m in operating loss.
InMed had C$18.0m in cash at 30 June and we believe this provides a runway into FY21. We now forecast the company will raise C$20m over the next two years to fund operations, which we model as illustrative long-term debt.
Business description
InMed Pharmaceuticals is a Canada-based biopharmaceutical company focused on manufacturing and developing cannabinoids. Its biosynthesis platform may be able to produce cannabinoids for less cost and with improved purity compared to currently used methods. The company is also developing a proprietary pipeline, including INM-755 for epidermolysis bullosa, a serious, debilitating orphan indication.
FY19 results update
InMed continues to be on track for initiation of the Phase I programme in healthy volunteers by the end of the year. The programme will consist of two separate trials (see Exhibit 1) that will be conducted in the Netherlands where there is less administrative burden to conduct a cannabinoid-based clinical trial. Trial 755-101-HV will consist of approximately 20 healthy volunteers with normal, intact skin and will evaluate the systemic and local safety, tolerability and PK of INM-755 cream. Trial 755-102-HV will have around eight healthy volunteers with small wounds to evaluate the local safety of the product, especially in people with broken skin (the small wounds will be created at the clinical site and will largely mimic the types of wounds typically seen in EB simplex patients). A Phase I/II in approximately 12–15 EB patients is expected to begin in Q121. Note that all these trials will be double blind and vehicle controlled. Importantly, the safety studies can be used as the basis for a clinical trial programme in other indications as INM-755 may have applications in other dermatologic indications involving inflammation, pain and itch.
The glaucoma programme is advancing. The company has chosen a new lead candidate (INM-088) due to superior preclinical results compared to the previous lead, INM-085, and its potential to treat other ocular disorders beyond glaucoma. Advanced preclinical and formulation development is expected to begin by the end of the year with key IND-enabling studies expected to begin in H120 following discussions with regulators. Exact timing for the initiation of human clinical studies is unclear but they could begin around H221.
Biosynthesis programme
With regards to the company’s E. coli-based biosynthesis programme, InMed has optimised several fermentation parameters that are part of the USP to maximise yield and has initiated the DSP.
The company is also exploring an alternative process (the exact nature of which is undisclosed) that may have advantages in terms of cost and yield (items of paramount importance in this industry). A decision on which process to move forward is expected by the end of the year. The selected process would then enter scale-up in H120 with GMP batch production expected in H220.
Valuation
We have slightly adjusted our valuation from C$255m or C$1.48 per basic share (C$1.14 per diluted share) to C$256m or C$1.48 per basic share (C$1.22 per diluted share). The valuation increase of rolling forward our NPV was offset by pushing back our expectations for initial revenues from the biosynthesis business to FY22 from FY21 (mainly for the sake of conservatism as FY22 begins in July 2021) and by lower net cash. The valuation per diluted share benefited from the fact that 13.9m options expired in July 2019 (an additional 16.6m warrants with a C$1.25 strike price expire in June 2020).
Financials
InMed reported an operating loss of C$9.6m in FY19 (the year ending 30 June 2019), up from C$5.4m in the prior year. R&D expenses were C$5.6m for the year, up from C$1.9m in FY18. We have increased our R&D estimates by C$1.3m in FY20 to C$11.3m as spending was higher than we initially expected in Q4. We also introduce FY21 estimates, which include C$11.7m in R&D spending and C$15.9m in operating loss.
InMed had C$18.0m in cash at 30 June and we believe this provides a runway into FY21. We now forecast the company will raise C$20m over the next two years to fund operations, which we model as illustrative long-term debt.