Incyte Corporation (NASDAQ:INCY) reported mixed results for the fourth quarter of 2018, wherein earnings missed expectations while revenues beat the same. The company reported earnings of 40 cents per share, which missed the Zacks Consensus Estimate of 43 cents but was up from 2 cents reported in the year-ago quarter.
Excluding milestone and contract, revenues came in at $528.4 million. Adjusting for the same, quarterly revenues were $468.4 million, up 25.2% year over year. Revenues beat the Zacks Consensus Estimate of $466.2 million.
Quarter in Detail
Total product-related revenues came in at $468.3 million, up 25% from the year-ago quarter. Of these, Jakafi revenues came in at $380.0 million, up 26% from the year-ago quarter, driven by strong patient demand. Also, Jakafi revenues beat the Zacks Consensus Estimate of $378 million. Net product revenues of Iclusig amounted to $19.1 million, down from $19.5 million in the year-ago quarter.
Product royalty revenues from Novartis AG (NYSE:NVS) for the commercialization of Jakafi in ex-U.S. markets grew 16% to $55.3 million. Olumiant’s product royalty revenues came in at $13.8 million.
R&D expenses were $274 million, flat with the year-ago quarter. SG&A expenses amounted to $97 million, up from $87 million in the prior-year quarter.
2018 Results
Revenues came in at $1.8 billion, which increased from $1.4 billion in the year-ago quarter and surpassed the Zacks Consensus Estimate of $1.70 billion. Earnings per share came in at $1.04 in 2018, missing the Zacks Consensus Estimate of $1.08.
2019 Outlook
The company expects Jakafi revenues of $1,580-$1,650 million. Iclusig revenues are expected to be $90-$100 million. R&D expenses are expected to be $1030-$1,100 million. SG&A expenses are anticipated to be $420-$470 million.
Pipeline Update
Incyte recently suffered a setback when the FDA extended the review period of the supplemental New Drug Application (sNDA) for Jakafi to treat patients with acute graft-versus-host disease (GVHD) who have had an inadequate response to corticosteroids. The sNDA was submitted in August 2018, and the FDA granted it both Priority Review status and Breakthrough Therapy designation.
However, the FDA extended the action date by three months to review additional data submitted by Incyte in response to the FDA’s information requests. The additional data have been determined by the FDA to constitute a major amendment to the sNDA. Hence, the new Prescription Drug User Fee Act (PDUFA) target action date is May 24, 2019.
Nonetheless, Jakafi is also being evaluated in patients with acute and chronic steroid-refractory GVHD who have an inadequate response to corticosteroids in the REACH2 and REACH3 clinical studies, respectively. Results are expected in the second half of 2019. Meanwhile, the global phase III GRAVITAS-301 trial on itacitinib as a treatment for patients with steroid-naïve acute GVHD is ongoing. Results from the study are expected in 2019.
The FDA has recently granted Breakthrough Therapy designation to pipeline candidate pemigatinib (FGFR) for the treatment of previously treated, advanced/metastatic or unresectable FGFR2 translocated cholangiocarcinoma. Incyte expects to submit an NDA for pemigatinib for the second-line treatment of patients with FGFR2 translocated cholangiocarcinoma in the third quarter of 2019.
A phase III trial on the cream formulation of Jakafi in patients with atopic dermatitis was initiated in December 2018. Data from the randomized phase II trial on Jakafi cream in patients with vitiligo are expected in 2019.
Incyte and partner Eli Lilly and Company (NYSE:LLY) announced positive top-line data from two late-stage studies, evaluating their oral JAK inhibitor, Olumiant (baricitinib) as monotherapy for moderate-to-severe atopic dermatitis (“AD”), a type of eczema. Data from the phase III studies — BREEZE-AD1 and BREEZE-AD2 — showed that the candidate met the primary endpoints of statistically significant improvements in patients compared to placebo, as measured by Investigator's Global Assessment for AD (“IGA”) score after treatment of 16 weeks. Full results are expected later in 2019.
Our Take
Incyte’s performance in the fourth quarter was mixed. Nevertheless, Jakafi sales continue to be impressive.
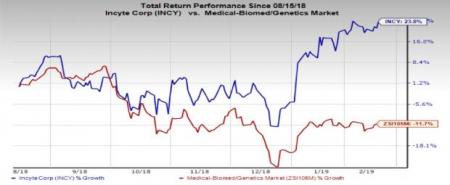
However, Incyte’s stock has lost 23.8% in the past six months compared with the industry’s 11.7% decline.
Pipeline setbacks continue to be a concern. The FDA’s extension of the review period of the sNDA for the label expansion of Jakafi to treat acute GVHD was disappointing. Earlier, the company suffered a huge setback with the failure of the phase III study, ECHO-301, as it was one of the most promising candidates for Incyte, evaluating epacadostat in combination with Merck’s (NYSE:MRK) Keytruda.
Zacks Rank
Incyte currently carries a Zacks Rank #5 (Strong Sell). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Will You Make a Fortune on the Shift to Electric Cars?
Here's another stock idea to consider. Much like petroleum 150 years ago, lithium power may soon shake the world, creating millionaires and reshaping geo-politics. Soon electric vehicles (EVs) may be cheaper than gas guzzlers. Some are already reaching 265 miles on a single charge.
With battery prices plummeting and charging stations set to multiply, one company stands out as the #1 stock to buy according to Zacks research.
It's not the one you think.
See This Ticker Free >>
Novartis AG (NVS): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Original post
Zacks Investment Research
