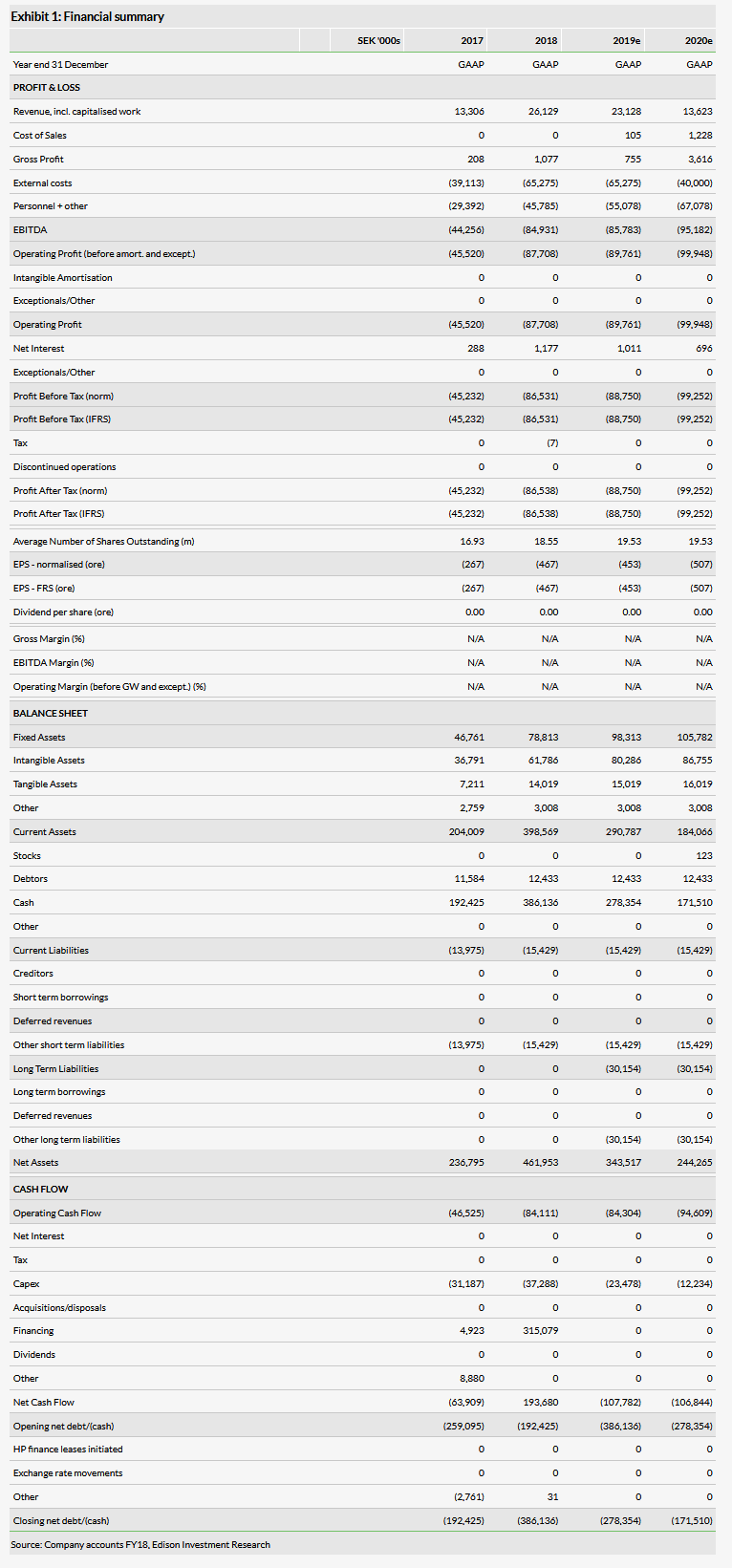
During 2019, Immunovia (ST:IMMNOV) will focus on optimising the IMMray PanCan-d test in preparation for the start of a commercial private testing service for pancreatic cancer (PDAC). The sample optimisation study should end in Q2. In H219, accreditation of the Swedish and US laboratories is required to enable a private PDAC test service by Q120. The 2,000 patient PanFAM-1 prospective clinical trial is still recruiting patients and might report primary data on early-stage PDAC detection in December 2019. Earlier-stage opportunities are: Type II diabetes, early symptoms of PDAC, lung cancer and rheumatoid arthritis. Our valuation remains at SEK3.5bn.
IMMray PanCan-d self-pay sales from Q120
The sample optimisation study might complete in Q219 after successfully sourcing enough fresh samples. The IMMray test itself is unaffected, but the algorithm may be adjusted. It seems unlikely to us that private sales can start before Q120; Immunovia will announce a schedule in Q2 including the three to five months needed for lab accreditation. Tests will be run in-house in Sweden and the US. Management guidance is for 2022 sales of SEK250–300m; c 50,000 privately paid tests at c US$600 per test. By 2024, guidance is for sales of SEK800–1,000m if insurance coverage is gained, based on PanFAM-1 data; c 164,000 tests.
Prospective study: PanFAM-1 might be late
The prospective PanFAM-1 clinical trial is essential for reimbursement. It is stated to complete its primary endpoint in December 2019 and end in March 2020 (21 January 2019, NCT03693378). The primary, 18-month endpoint is: “Demonstrate that the IMMray PanCan-d test is equal or better than standard imaging for early detection of PDAC in asymptomatic high-risk individuals.” As two new centres were added in Q1, making 17, recruitment is ongoing. Accordingly, we question if published dates can be met as the trial cannot end until 18 months after the final individual enrols unless PDAC incidence rates are much higher than the literature suggests. If recruitment ends by June 2019, the endpoint might be in H220-Q121. The published (Mellby et al 2018) sensitivity is 93%, specificity 95%, accuracy 96%.
Valuation: DCF SEK3.5bn or SEK177 per share
Our valuation remains at SEK3.5bn pending the schedule update promised in Q2. We assume the first sales of PanCan-d are in Q120. Edison’s 2028 peak sales forecast is unchanged at SEK2.5bn. There are earlier-stage opportunities in patients with potential PDAC symptoms, in screening Type 2 diabetics for pancreatic cancer (about 0.5% incidence on diagnosis), detecting lung cancer and rheumatoid arthritis. Of these, the potential PDAC study has been extended in a London-based study and the test could be useful if it rapidly rules out PDAC.
Business description
Immunovia is a Swedish company specialising in diagnostics for oncology and autoimmune diseases. Its main product is IMMray PanCan-d, an antibody microarray based on its proprietary IMMray platform. A prospective trial in 2,000 people at high risk of familial pancreatic cancer is ongoing. The company expects to make self-pay service-test sales from 2020.