Hutchison China MediTech (NASDAQ:HCM) has announced positive data that key late-stage asset surufatinib met the primary endpoint of PFS in non-pancreatic at the Phase III interim analysis. This translates to an earlier than expected China NDA submission (H219) and the potential launch of HCM’s first un-partnered asset (early 2021). In China, partner Lilly has launched Elunate (fruquintinib) capsules. Early sales look promising and its potential inclusion on the China NRDL later this year will be definitive to the China opportunity. However, failure of fruquintinib monotherapy in third-line NSCLC and the changes in strategy to savolitinib in RCC has negatively affected our valuation. We forecast two further product launches on the horizon in 2021/2022 (China launch of fruquintinib in gastric cancer and global launch of savolitinib in NSCLC). HHHL has completed a secondary offering of ADSs, which has reduced its holding to 51.15% (from 60.2% previously). We see this as a significant positive for HCM as it increases the free float, potentially leading to better liquidity. We value HCM at $5.6bn (£6.72/share) vs $6.5bn previously.
Business description
Hutchison China MediTech is an innovative China-based biopharmaceutical company targeting the global market for novel, highly selective oral oncology and immunology drugs. Its established commercial platform business continues to expand its outreach.
Elunate launched, surufatinib to follow shortly
Elunate is now being commercialised in China (third-line CRC) by partner Eli Lilly (NYSE:LLY) (Lilly), a major inflection point for HCM and validation of its R&D philosophy. The positive interim readout of surufatinib (met primary endpoint of PFS) in the SANET-ep Phase III trial in non-pancreatic NET (trial was able to be stopped early) is of huge importance and these data will support a China NDA submission in late 2019. The years 2021/2022 are pivotal; partner AstraZeneca (AZN) could launch savolitinib in China for NSCLC (MET exon 14) and could become HCM’s first asset to launch internationally (2022) in combination with Tagrisso for NSCLC.
Combination strategies to drive future growth
HCM has accelerated global (ex-China) development of its wholly owned assets (fruquintinib, surufatinib, HMPL-523 and HMPL-689). Ongoing advances and thus changes in treatment paradigms in many oncology indications mean that combination therapy is the best approach to treat resistant cancers. With this in mind, HCM has announced four new PD-1 collaborations for fruquintinib and surufatinib. HCM/AZN are focusing savolitinib’s ex-China strategy on its use in combination with Tagrisso in MET-driven NSCLC, a blockbuster opportunity.
Valuation: $5.6bn (£6.72/share)
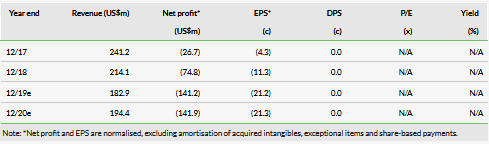
We value HCM at $5.6bn (£6.72/share) vs $6.5bn previously. Our revised valuation takes into account the success of the SANET-ep Phase III trial, failure of fruquintinib monotherapy in third-line NSCLC and the changes in strategy to savolitinib in RCC. We have additionally adjusted our product timelines to include combination strategies across the portfolio and revisited our R&D and S&M costs.
Investment summary
Our long-term investment cases rests on HCM’s ability to develop and commercialise its assets in both China and the rest of the world (RoW). HCM is rapidly investing in international expansion with five drug candidates in or about to enter global clinical trial development. Fruquintinib, savolitinib and surufatinib lead the pack with the earlier-stage spleen tyrosine kinase (Syk) and PI3K inhibitors coming to the fore. The launch of Elunate (fruquintinib) in China (November 2018) is a significant milestone for HCM, while the successful and early readout (June 2019) of surufatinib in the SANET-EP Phase III trial in non-pancreatic neuroendocrine tumours (NET) supports a China New Drug Application (NDA) submission in late 2019 and a potential launch early 2021. Key to HCM’s global ambitions is SAVANNAH, the registrational study of savolitinib in combination with Tagrisso in non-small-cell lung cancer (NSCLC) patients. Clinical trials design and execution will become ever more critical in the fast-changing treatment landscape for oncology and we believe HCM remains committed to driving forward only assets and strategies that will have a material impact on returns. Combining its pipeline with approved and novel targets will be of great importance in the future of cancer treatment paradigms.
Recent market update: Increased free float
Hutchison Healthcare Holdings Limited (HHHL) has completed a secondary offering of ADSs, which has reduced its holding to 51.15% (from 60.2% previously). We see this as a significant positive for HCM as it increases the free float, potentially leading to better liquidity. We note this has had no effect on shares in issue and does not dilute any current shareholders. In connection with this offering, HCM, its officers and directors, in addition to HHHL have agreed to a 90-day lock-up on sale or transfer of ordinary shares. As a result of this the proposed Hong Kong (SEHK) listing is now on hold. However, HCM has reiterated its commitment to the listing and a concurrent global offering of ordinary shares and may still pursue both following the cessation of the lock-up. An eventual listing on SEHK will be determined on a number of factors including, but not limited to, management decisions and market conditions. In our view, a capital raise on existing markets (AIM/NASDAQ) still remains likely in the next 12–24 months, however, cash resources as last reported (at 31 March 2019) of $391.2m are more than sufficient to enable current operational and strategic goals.
Valuation: $5.6bn (£6.72/share)
We value HCM at $5.6bn (£6.72/share) vs $6.5bn previously. We use a risk-adjusted net present value (NPV) method to discount future cash flows for the innovation platform (IP) (valuation of $4,004m). We have adjusted our valuation to reflect the success of the SANET-ep Phase III trial, the failure of fruquintinib monotherapy in third-line NSCLC (FALUCA), the cessation of enrolment in the SAVOIR (savolitinib monotherapy in the papillary renal cell carcinoma (PRCC)) study and the discontinuation of the clear cell RCC monotherapy trial (component of CALYPSO trial) in favour of combination strategies. Additionally, launch dates for key assets have been pushed back, while time to peak sales in China has been brought in line with our assumptions for the RoW and peak sales assumptions have been updated. We use earnings-based multiples for HCM’s commercial platform (CP) (subs and JVs), applying a 20.4x multiple on our forecast 2019 net attributable profit (equity in earnings of equity investees, net of tax) for the JVs of $39.2m yields a valuation of $800.4m. We note that the significant increase in our terminal value from £0.56/share to £1.22/share is driven by the adjustment of time horizons, notably the terminal value now captures a greater proportion of key product sales. Adding April 2019 net cash and netting out unallocated costs results in a value of $5.6bn.
Sensitivities: Changing treatment landscapes
HCM is subject to the usual biotech and drug development risks, including clinical development delays or failures, regulatory risks, competitor successes, partnering setbacks, and financing and commercial risks. We note that the rapidly advancing oncology treatment landscape remains a key sensitivity with potential for clinical trial programmes to become irrelevant while in progress (as evidenced by the cessation of the SAVIOR Phase III study) or for early efficacy readouts (eg surufatinib SANET-ep) to bring forward regulatory submissions and potential launch dates. In China, regulatory and reimbursement changes mean drugs have a potential faster route to approval and reimbursement than historically; China is becoming an increasingly important market for innovation drugs given supportive government policies and population size.
Financials: Funded to inflection points
Full-year 2018 results published in March 2019 highlighted robust growth in net profit attributable to the commercial platform division in China, coupled with clinical progress within the IP portfolio. HCM reported available cash resources of $391.2m (at 31 March 2019) at the group level (cash and cash equivalents including short-term investments of $271.9m, and unutilised bank borrowing facilities of $119.3m). In May 2019 HCM entered into a credit agreement with HSBC for an additional $51.3m unsecured credit facility. This, combined with the net profit generation from the CP division, means that HCM is well funded to key value inflection points (NDA submissions for savolitinib, surufatinib and fruquintinib in 2019–2021), even taking into account the considerable rise in R&D expenses seen in 2018, which we expect to continue to rise in the near term.
China: A stepping stone to global ambitions
With HCM’s financial strength and its drive to retain the economic value of its assets, the next steps for the company are to commercialise its own assets in China then internationally (outside of current partnerships). The launch of Elunate (HCM’s first internally developed asset) in China is a significant milestone for HCM and gives us confidence in the company’s ability to execute on its R&D philosophy of building first- or best-in-class molecules with lower toxicity profiles to enable combination-based strategies for the treatment of cancers. We expect next approvals in China for surufatinib in NET, savolitinib in NSCLC (MET Exon 14 skipping) and fruquintinib in gastric cancer.
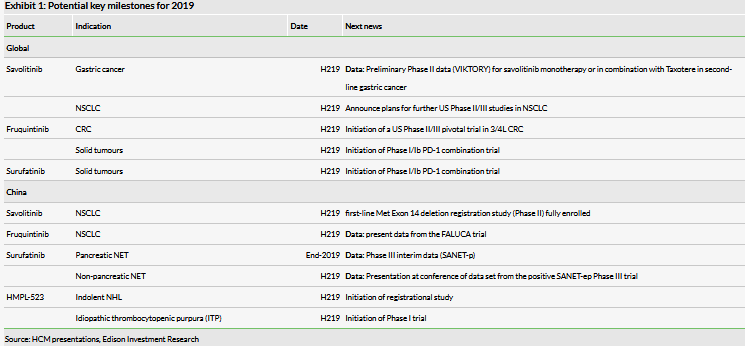
Outside China, HCM is now accelerating its operations as it transitions into a global biopharma; importantly, clinical and regulatory teams are now fully operational in the US and EU. HCM’s first global approval (we forecast launch in 2022) in the US/EU could be for a savolitinib combination with AZN’s Tagrisso in epidermal growth factor receptor mutant (EGFRm), MET+ NSCLC. Exhibit 1 highlights the numerous clinical milestones, both in China and globally, likely in 2019.
China: Launch of Elunate marks the start of things to come
Eli Lilly launched Elunate (third-line colorectal cancer (CRC) patients) in November 2018 (at 31 March 2019 HCM reported $0.978m in royalties, implying $6.5m Elunate end-user sales in Q119) and the next development focus is on Phase III gastric cancer (FRUTIGA), for which approval could occur in 2021/22.
The recent successful efficacy read-out of surufatinib in the SANET-EP Phase III trial in non-pancreatic NET was earlier than we had anticipated and positions surufatinib as the next internally developed R&D asset to launch in China; HCM will hold a pre-NDA meeting with the China National Medical Products Administration to discuss the preparation of the NDA. Additionally, in China, registration studies continue for savolitinib in MET Exon 14 deleted NSCLC patients (primary data early-2020) and in surufatinib in pancreatic NET (interim data end-2019). Elunate and surufatinib mark the start of multiple potential drug launches in the near term.
Global: Infrastructure in place as clinical programme expands
Our analysis concludes that much higher economic value resides in the strategy to develop and commercialise HCM’s own assets, in particular with respect to fruquintinib (ex-China), surufatinib, epitinib, HMPL-523 and HMPL-689. HCM is now accelerating its global operations as it transitions into a global biopharma with clinical and regulatory (C&R) teams now fully operational in the US and EU. HCM plans to expand its US and EU C&R operations (headquartered in New Jersey, US) to 30 employees by end 2019 and further expansions should be expected in subsequent years as HCM builds ex China commercial infrastructure ahead of the potential launch of non-partnered assets surufatinib and fruquintinib (subject to positive clinical trial data and regulatory approval).
Global development is currently focused on savolitinib (with partner AZN) with multiple late-stage trials in progress, including SAVANNAH (second-line/third-line EGFRm, Tagrisso refractory, MET positive NSCLC with Tagrisso), CALYPSO (papillary and clear cell RCC with/without PD-L1 Imfinzi), and VIKTORY (MET-positive gastric cancer). Additionally, a proof-of-concept trial has initiated in Canada testing savolitinib in MET positive prostate cancer.
HCM’s first approval (we forecast launch in 2022) in the US and EU could be for a savolitinib plus Tagrisso combination in MET+ NSCLC patients. AZN’s Tagrisso has quickly become the standard of care in first-line NSCLC patients who have an EGFR mutation (EGFRm). Following Tagrisso treatment, the most common mutation is c-MET and AstraZeneca sees savolitinib as key to its strategy for MET driven NSCLC. In addition to savolitinib, HCM has already commenced the global development programme for fruquintinib (CRC US registration study in planning based on China FRESCO data and completed US Phase I/II data) and surufatinib (US Phase I/II pancreatic NET ongoing). Additionally, development programmes for HMPL-523 (Syk inhibitor) and HMPL-689 (PI3Kδ inhibitor) in indolent NHL are expected to start Phase I/Ib trials shortly. We forecast global launches from 2022.
Worldwide: Combinations the focus across regions
HCM’s R&D efforts has successfully generated a portfolio of candidates that address existing, well-validated targets with lower toxicity profiles as a result of target selectivity. Cleaner toxicity profiles should enable drug combination as often lack of tolerability leads to failure. Combinations of targeted therapies (TKI (tyrosine kinase inhibitor), monoclonal antibodies and immunotherapies) and chemotherapy is increasingly becoming the best approach to treating the complex and constantly mutating disease that is cancer.
In the last 12 months HCM has signed four co-development collaborations for fruquintinib and surufatinib in combination with a variety of China developed PD-1 (programmed cell death protein-1) monoclonal antibodies. Advances in the field of immune oncology in the last few years and the increasing understanding that different classes of cancer drugs (targeted therapies and checkpoint inhibitors) may act synergistically together means that the industry needs to keep up the pace and ensure that trial design and combinations address the most pertinent question of all: which drug regimen is best for a given cancer. HCM’s partnership with AstraZeneca means that savolitinib’s international focus is on combinations with a number of AZN’s oncology drugs including Tagrisso and Imfinzi. The renegotiated deal with Lilly for fruquintinib has given HCM freedom to explore different line extensions and combinations through non-restrictive collaborations.
Pipeline overview
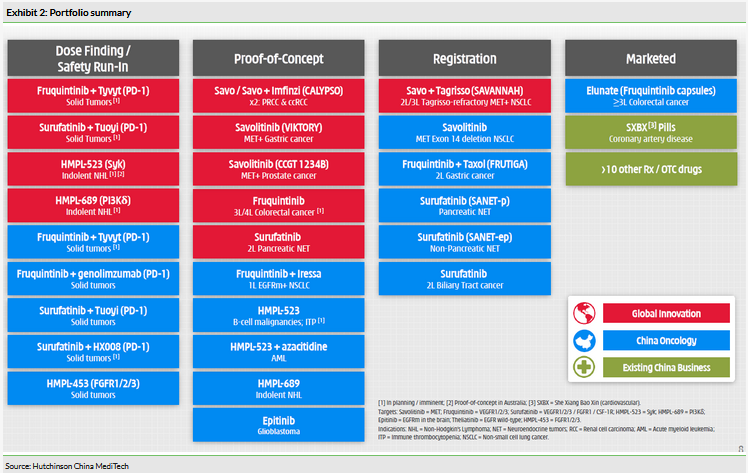
HCM has multiple tyrosine kinase inhibitors in clinical development, with eight assets in the clinical phase of development across over 30 trials globally, including five registration studies that are expected to read out over the next 24 months. Exhibit 2 provides an overview of the pipeline and segments the asset opportunity by China NDA opportunities (blue) and the international drug development programmes (red). Assets marketed through China CP are in green.
In the next sections we discuss in turn:
- Elunate/fruquintinib capsules: launched with partner Lilly in China for mCRC in November 2018. FRUTIGA is another trial investigating combination with Taxol in gastric cancer; top-line data are expected in 2020. With FALUCA missing its primary endpoint in NSCLC, HCM will now explore PD-1 combinations that could drive earlier use.
- Savolitinib: two pivotal studies in NSCLC are ongoing – in China for MET Exon 14 skipping and globally for a Tagrisso savolitinib combination (SAVANNAH). The PRCC strategy will focus on a combination with a PD-L1 inhibitor following changes in renal cancer treatment paradigms.
- Surufatinib: China NDA filing in 2019 for non-pancreatic NET. This would be the first un-partnered launch of a HCM asset.
- HMPL-523 and HMPL-689: a Phase I/Ib study in Australia and China has expanded its enrolment (blood cancers). IND applications for both HMPL-523 and HMPL-689 have been accepted for US and EU clinical development to start.
Elunate: Innovation into commercialisation
The launch of Elunate (fruquintinib capsules) for metastatic colorectal cancer (third-line and above) by partner Lilly in November 2018 was a defining moment for HCM, as the first of its IP assets to launch in China and the first China discovered and developed new drug for oncology to have full approval and reach the market. At 31 March 2019 HCM reported $0.978m in royalties, implying $6.5m Elunate sales in Q119. Elunate has been priced at $3,300 per monthly cycle (RMB21,960) and a patient-access programme has been implemented to widen availability in China (patients pay for the first, second and fifth cycle). HCM’s target patient population for third-line CRC is ~55,000–60,000 patients (third line is 15% of incidence in China of 380,000 patients per year). Importantly, inclusion on China’s National Reimbursement Drug List (NRDL) will define the opportunity given full-scale reimbursement nationally. Inclusion on the list is conceivable in H219 as the Chinese government agencies are reviewing inclusions and exclusions on the list much more frequently than in the past.
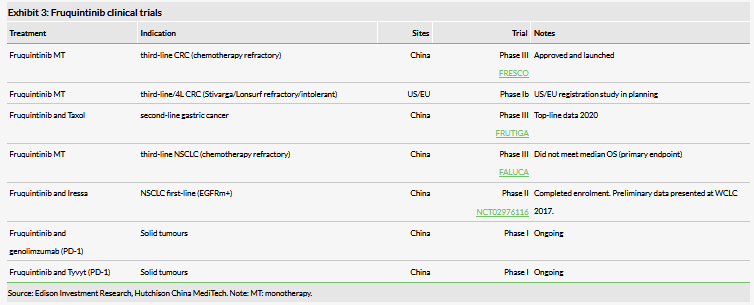
Exhibit 3 highlights the status of Fruquintinib clinical trials in China and internationally in a broad range of oncology indications.
Positive renegotiated Eli Lilly deal terms
In December 2018, HCM and Eli Lilly renegotiated their 2013 agreement on Elunate. Under the terms of the new deal (Exhibit 4), HCM has acquired the rights to determine for which indications (LCI: life cycle indications) it will further develop Elunate. HCM will now pay the full development costs of any future clinical development in China and in return will benefit from higher economic value retention including both higher approval milestones and royalties. HCM additionally now has the option to co-promote Elunate in China. On the occurrence of non-fruquintinib related Eli Lilly commercial action (which we assume is related to a China launch of Cyramza), HCM will be able to promote Elunate in provinces that represent 30–40% of sales of Elunate. Lilly will pay HCM a service fee for all promotional activities in the HCM regions. While HCM already has a significant salesforce in China, the co-promotion deal enables HCM to gain experience in commercialising its own in-house developed products and expand its oncology salesforce prior to launching other pipeline assets. Importantly, HCM retains the full development and commercial rights to fruquintinib outside of China (assuming regulatory approvals are achieved).
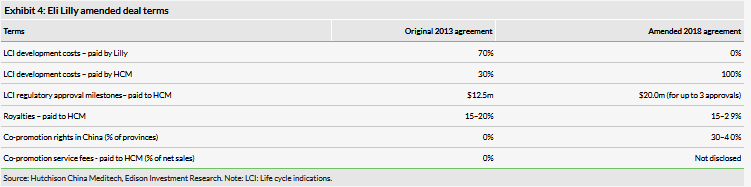
The renegotiated deal terms on Elunate (fruquintinib) are a major positive in our view as these give HCM freedom of control on development of the asset into multiple oncology indications, not just as a monotherapy but also in combination therapies, the newly announced collaborations with Innovent Biologics and Genor Biopharma signal this. With the simplification of the LCI decision-making process from two parties to one, we expect HCM will accelerate Elunate’s commercial development in China beyond the indications currently in development, particularly in immunotherapy combinations.
FRUTIGA top-line data in gastric cancer expected in 2020
The National Central Cancer Registry of China reports an incidence of 679,000 gastric cancer cases and 498,000 gastric cancer deaths in China in 2015. Most Chinese gastric cancer patients receive chemotherapy and few targeted therapies are approved, with limited salvage treatments in third line and above. HCM has initiated a pivotal Phase III (FRUTIGA) combination trial (second line) evaluating fruquintinib and established chemotherapy agent Taxol (paclitaxel) for the treatment of advanced gastric cancer patients (n>500) who have progressed after first-line standard chemotherapy (5-fluorouracil and platinum doublets). If the FRUTIGA Taxol combination data are positive, this would enable fruquintinib use in earlier lines of gastric cancer. The full top-line data are expected in 2020. The primary efficacy endpoint for FRUTIGA was overall survival (OS); secondary endpoints included PFS (progression-free survival), ORR and DCR.
FALUCA primary OS endpoint not met in NSCLC
In November, HCM announced data from FALUCA, HCM’s China-based Phase III trial for fruquintinib monotherapy in third-line NSCLC (after failure on two lines of systemic chemotherapy). The trial missed its primary endpoint of improving overall survival compared to placebo. The full dataset is yet to be presented (has been submitted for publication at a scientific conference), but statistically significant improvements were observed across all secondary endpoints (data not disclosed): PFS, duration of response (DoR) and objective response rate. Without a fully analysed dataset, and ascertaining the underlying cause for missing this endpoint, the future direction for fruquintinib monotherapy in third-line NSCLC is unclear. What is becoming clear is that advances in cancer therapy, including targeted therapies and in the more nascent field of immunoncology, are changing treatment paradigms and this has implications for ongoing clinical trials that were designed and initiated a few years back (including FALUCA). Higher than expected survival rates seen in placebo arms could be a function of patients receiving other therapies at higher lines not accounted for when FALUCA was initially designed skewing the results. Thus we now assume that any future development in NSCLC is focused on combination therapies, particularly immunotherapy combinations (see below). While there are no planned combinations of fruquintinib with Tagrisso and/or savolitinib in NSCLC, we believe any combinations could be synergistic and provide a point of differentiation for fruquintinib.
PD-1 combination the focus for future development
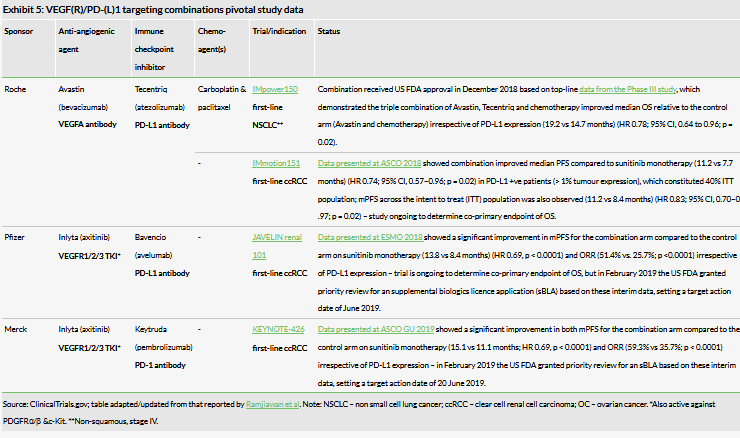
Targeted therapies and immunotherapies (monotherapy) have improved patient outcomes across many tumour types; combinations that work synergistically could enhance results further. HCM has developed Elunate to compete in the ~$16bn vascular endothelial growth factor receptor (VEGFR) market, which is dominated by Roche’s Avastin (bevacizumab), a VEGF-A inhibitor that is an intravenously administered monoclonal antibody (Roche reported 2018 sales of $6.9bn). Avastin inhibits VEGF A protein, whereas fruquintinib targets VEGF receptors.
In December 2018 the US FDA approved the triple combination of Tecentriq, Avastin and chemotherapy (carboplatin and paclitaxel) for first-line NSCLC (non-squamous), which is not driven by EGFR or ALK mutations (Exhibit 5). This serves as a strong validation that a combination of an anti-angiogenic agent (Avastin) with an immune checkpoint inhibitor (Tecentriq) can have added benefit to patients with NSCLC; this highlights the potential for fruquintinib and surufatinib (which both inhibit VEGFR1/2/3) in combination with PD-1 antibodies. Similarly, data from three pivotal studies investigating analogous combination therapies for RCC (IMmotion151, JAVELIN renal 101 and KEYNOTE426) look promising (Exhibit 5) and support combination use.
In November, HCM entered into a range of collaborations to test surufatinib and fruquintinib with PD-1 inhibitors. For fruquintinib, HCM has a global partnership with Innovent Biologics and its PD-1 inhibitor sintilimab (IBI308) and a China-focused collaboration with Genor BioPharma and its PD-1 inhibitor genolimzumab (GB226).
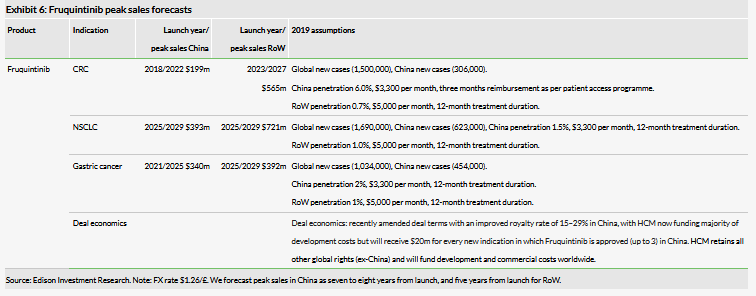
Fruquintinib (Elunate) peak sales assumptions
We have revisited our fruquintinib (Elunate) expectations, in the light of the differing pricing strategy to our expectations, and the disappointing FALUCA data in NSCLC monotherapy and move to combination strategy in this indication. We forecast global peak sales for fruquintinib of $2.6bn across all indications under investigation (CRC, NSCLC and gastric cancer). The magnitude of fruquintinib’s success in part will depend on the global opportunity; we forecast RoW launches from 2023. In China Elunate gross pricing is $3,300/month, but as a result of HCM’s Patient Access Programme, the median cost for Elunate over an average five months of use is $1,980/month. We now forecast peak sales of $199m in China vs $149m previously. We believe this pricing strategy and patient access programme could increase hospital formulary access and we assume that Elunate will be added to China’s NRDL in the near term, inclusion will be a major growth driver.
Savolitinib: Striving for combination success
Since our last report was published, much clinical progress in the field of MET-driven cancers has occurred. Foremost is changes in the renal cancer treatment paradigm. We had previously anticipated that savolitinib monotherapy for PRCC would be the first launched indication, potentially in late 2019 through a breakthrough approval in the US. However, recent paradigm shifts in the treatment of renal cell carcinomas are moving towards combinations with a PD-1 inhibitor. As such, AZN has suspended the Phase III monotherapy study in PRCC (SAVOIR) and future development will likely focus on a combination with AZN’s PD-L1 inhibitor Imfinzi (see below); this decision also took into account data from the MES (molecular epidemiology study) which showed smaller than expected applicable MET+ status.
In the field of lung cancer, AZN’s Tagrisso is raising the bar as it moves into the first-line setting in EGFR mutation-positive NSCLC, reporting sales of $1.9bn in 2018, its second full year on the market. The implication here is savolitinib’s largest opportunity could be in combination with Tagrisso in EGFRm MET+ NSCLC patients as MET mutations are the biggest driver in Tagrisso resistance. The savolitinib/Tagrisso combination could rewrite the second-line/third-line treatment paradigm and our forecast peak sales could be conservative. Following the encouraging data seen from the TATTON study, AZN and HCM in December 2018 initiated the global registrational study SAVANNAH for Tagrisso refractory NSCLC patients. HCM’s partnership with AZN remains crucial to the development of savolitinib and our forecast peak sales.
Savolitinib is a highly selective inhibitor of the c-Met signalling pathway and targets patients with resistant cancers whose tumour type tests positive for MET mutation, amplification or over expression. Savolitinib is hypothesised to have a greater beneficial impact on c-Met-driven tumours than approved multi-kinase inhibitors, particularly in EGFR-resistant patient subgroups. C-Met (or hepatocyte growth factor receptor/HGFR) is a signalling pathway, dysregulation of which mediates proliferation, apoptosis and migration in many tumour types including NSCLC, RCC and gastric cancer. This aberrant functioning can arise through c-Met gene amplification, c-Met over expression and gene mutations and the extent of each will differ by tumour type and patient. A key challenge in the area is identifying which biomarker and thus which diagnostic is most relevant to select the appropriate patients for MET targeted treatment as monotherapy and as combinations. Exhibit 7 highlights the incidence of MET dysregulation in NSCLC; the type (eg gene over expression, HGF expression, gene amplification, gene mutation) and the relevant biomarker for testing.
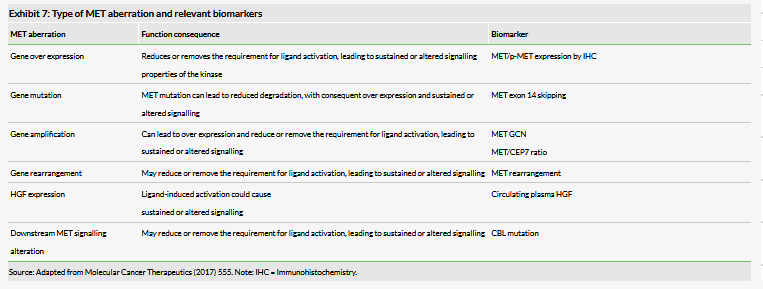
We highlight that the field is still in many ways in its infancy as companies such as HCM and Novartis (capmatinib) are at the forefront of developing c-MET inhibitors. In late 2016 Phase III trial results of onartuzumab plus erlotinib in NSCLC did not prove clinical benefit over the comparator arm and it is thought that the clinical eligibility of patients based on MET over expression as measured by IHC may not be sufficiently sensitive and specific as a diagnostic tool to identify MET positivity.
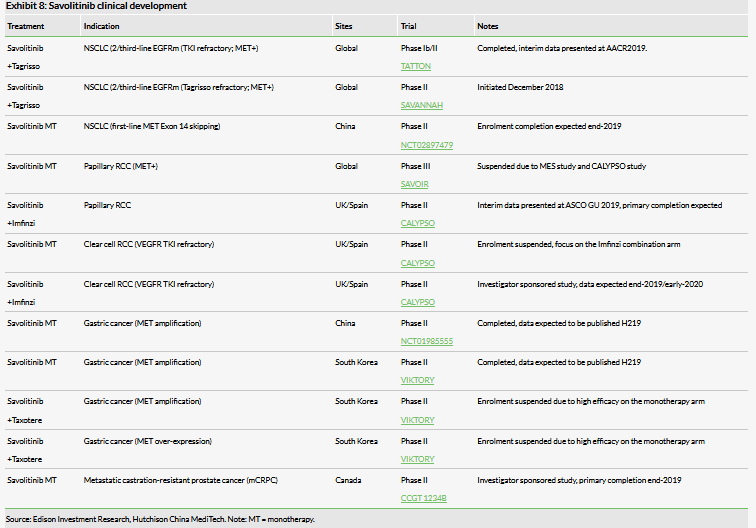
Tagrisso combination could rewrite NSCLC paradigm
In the NSCLC setting savolitinib is being evaluated as a monotherapy in the MET Exon 14 skipping China Phase III registration study, in combination with Tagrisso (global TATTON and SAVANNAH studies). We highlight the largest opportunity is increasingly becoming the Tagrisso plus savolitinib combination in resistant driven EGFRm+ MET + patient populations. In our view savolitinib’s first approval for NSCLC ex China will likely be in combination with Tagrisso (AZN) for MET+, T790M± patients who have progressed following Tagrisso. This specific subset of patients has an unmet medical need and, although we forecast a 2022 launch, BTD award could lead to earlier approval and launch.
AZN has presented analyses of FLAURA (first-line Tagrisso) and AURA3 (second-line Tagrisso) data examining mutation status (via plasma sampling) of patients who become resistant to Tagrisso. While no known mechanism of resistance was identified in 60% of patients in AURA3, MET amplification was identified as an acquired resistance mechanism in 19% of patients and 15% of patients in FLAURA. Understanding the mechanism of acquired resistance following Tagrisso therapy is a key clinical question to inform the next treatment choice. AURA3 suggests that patients could benefit from addition of savolitinib to Tagrisso in MET +ve patients as second-line therapy.
Critical for the future clinical trials planned for savolitinib is understanding the width and breath of MET alteration and how it can be best detected (tissue biopsy vs plasma testing). More recently it has been recognised that MET amplification in NSCLC is implicated in acquired resistance to EGFR inhibitors in ~20% of cases with EGFR inhibitor resistance. A 2018 analysis of plasma samples from Tagrisso’s AURA3 trial identified MET implication to be the cause of Tagrisso resistance in 19% of these patients in second-line and above. Data from the Tagrisso FLAURA trial suggest that MET amplification is implied in 15% of cases in first-line. In both cases, plasma samples were measured and it is thought that the frequency of MET amplification could be higher in tissue samples. This provides further therapeutic rationale for combinations of MET inhibitors with EGFR inhibitors to resistant NSCLC and the question is how high up in the paradigm savolitinib can be used (in combination with Tagrisso). The SAVANNAH and ORCHARD (see below) studies that are underway should shed light on these questions.
TATTON data: Consistent with interim analysis
In 2014 AZN initiated the TATTON study, a multi-arm Phase Ib/II study to test the suitability of an array of therapies with Tagrisso (osimertinib) in EGFRm+ NSCLC patients who have progressed following treatment with EGFR TKIs. Patients who had cancer progression driven by MET amplification (identified by either NGS [next generation sequencing], FISH [fluorescence in situ hybridization] or IHC) were placed on a combination regime of savolitinib and osimertinib, stratified into two cohorts based on whether they had progressive disease (PD) while on a first- or second-generation EGFR TKI (ie Iressa/Tarceva) or a third-generation EGFR TKI (ie osimertinib). Data from these two dose-expansion cohorts were presented at AACR2019 (Cohort 1 and Cohort 2), and reaffirmed the tolerable safety profile and promising efficacy data from the preliminary analysis that was presented at WCLC2017.
Following the encouraging data seen from these arms of the TATTON study, AZN and HCM in December 2018 initiated the global registrational study SAVANNAH, a Phase II, single-arm trial assessing the efficacy of Tagrisso in combination with savolitinib for patients with EGFRm, MET-amplified, locally-advanced or metastatic NSCLC who have progressed, following treatment with Tagrisso. Primary data is expected in 2021. Likewise, AZN is planning to conduct ORCHARD, a Phase II, multiple-arm trial looking to identify the drivers of resistance in NSCLC patients who have progressed following first-line treatment with Tagrisso; this should act as a feeder to SAVANNAH and aid in patient recruitment.
NSCLC first China NDA submission in 2021
An estimated 2–3% of newly diagnosed NSCLC patients have a specific mutation known as MET Exon 14 skipping (Exon 14 of the MET gene is not functioning or deleted) leading to c-MET over expression. In China, HCM estimates this to be 10,000 patients. A registrational Phase II clinical trial is underway in such patients who have progressed on prior chemotherapy, or who are unable to tolerate additional rounds of chemotherapy. Although the patient size is relatively small, this indication could be savolitinib’s first NDA in China. Interim data were presented at AACR2019 and of the 31 evaluable patients, 17 had a confirmed partial response (ORR of 54.8%), 12 patients had stable disease and two patients showed disease progression (DCR of 93.5%). Savolitinib was generally well tolerated, with only 14.5% (six of 41 patients) discontinuing treatment due to treatment emergent adverse events, mainly drug-induced liver toxicity (three patients); six patients died on treatment, one of which was suspected to be treatment related (tumour lysis syndrome). Trial recruitment (in China) is expected to complete in 2019 and completion/primary data can be expected in 2020, potentially with a Chinese NDA submission in 2021. We forecast launch in this indication in 2022. In the longer term in China, we believe a savolitinib plus Tagrisso combination will expand use in other subsets of NSCLC.
Data presented at ASCO2019 have highlighted the competitive landscape emerging (globally) for targeted treatments of MET Exon 14 skipping NSCLC patient populations; both Merck’s c-Met inhibitor tepotinib and Novartis’s capmatinib demonstrated impressive ORR when used in treatment naïve patients (59% and 68% respectively) and as 2/third-line treatments (45% and 41% respectively).
Kidney cancer: ICI combinations are game changing
The savolitinib registration strategy for kidney cancers, in particular PRCC, is being reassessed, taking into account findings from the molecular epidemiology study (MES) in PRCC patients and the realisation that treatment paradigms for RCC have shifted with the approval of PD-(L)1 inhibitors and increasing use of VEGFR inhibitors. As a result AZN and HCM have stopped enrolment of patients into SAVOIR, a Phase III global registration study evaluating savolitinib monotherapy (vs sunitinib monotherapy) in MET-driven PRCC patients. Successful commercialisation of savolitinib in kidney cancer (both ccRCC and PRCC) now hinges on CALYPSO, an investigator sponsored Phase II study combining savolitinib with AstraZeneca’s PD-L1 inhibitor Imfinzi (durvalumab).
In clear cell renal cell carcinoma (ccRCC) PD-(L)1 immune checkpoint inhibitor are revolutionising the treatment landscape ; the combination of MET inhibition with PD-(L)1 inhibition could have utility in this space, as underlined by promising preliminary data from CALYPSO presently at ASCO GU 2019.
Savolitinib: Combined peak sales potential of $4.3bn
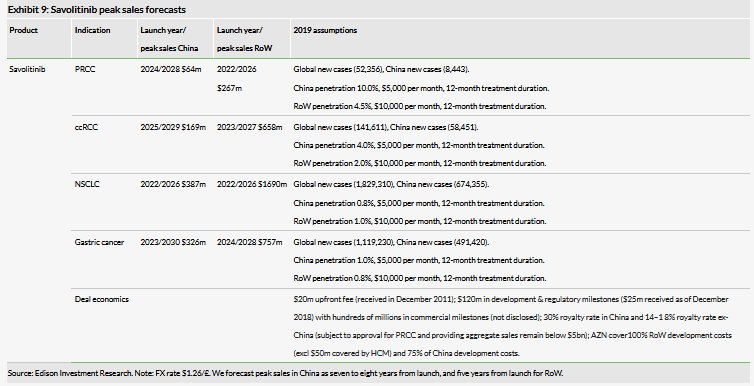
We forecast global peak sales for savolitinib of $4.3bn (PRCC, ccRCC, NSCLC and gastric cancer indications), lowered from our previous $5.9bn expectation. We have re-evaluated our assumptions following changes in market dynamics, and changes in cancer treatment paradigms. In our view savolitinib’s first RoW approval for NSCLC will likely be in combination with Tagrisso (AZN) for MET+, T790M± patients. Additionally, we have reassessed our expected launch dates (and thus peak year of sales) and have pushed back launch years across indications (Exhibit 9), notably with the first indication launch (in second-line/third-line NSCLC) now forecast for 2022.
Surufatinib: Positive Phase III data, China NDA H219
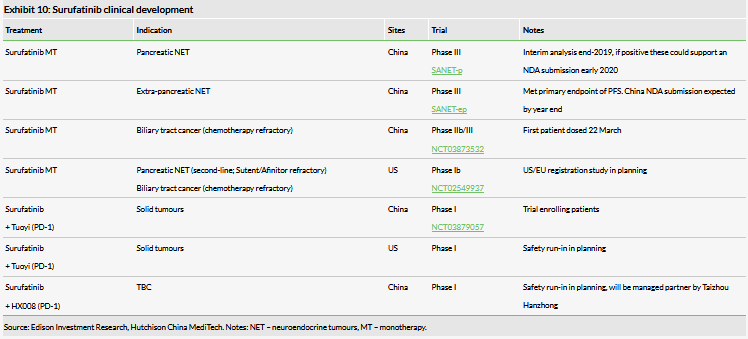
Surufatinib (previously known as sulfatinib) could be the first of HCM’s non-partnered assets to reach the China market in early 2021. The strategy for surufatinib was recently validated as positive interim data were announced in the SANET-ep Phase III trial testing surufatinib in non-pancreatic NET China patients. This was based on the trial meeting its primary endpoint of progression-free survival. The data support a China NDA submission in late 2019 and we forecast a launch in 2021. However, a launch could occur in late 2020. It previously took 15 months from submission of the NDA to approval for fruquintinib (Elunate). However, three months was to gain GMP certification for HCM’s Suzhou manufacturing plant, which should not be necessary for surufatinib. If the NDA is submitted within four months (October 2019) of the recent announcement of the top-line data (as was the case for fruquintinib), then we believe a launch in late 2020 is possible. However, predicting review timelines for the China FDA is notoriously difficult and this timeline could slip. As such, we currently retain our 2021 launch date. Additionally, a pivotal China Phase III study (SANET-p) in pancreatic NET is ongoing with data expected by the year end. If positive, this could further widen the market potential of surufatinib.
NETs are cancers that arise out of cells of the endocrine and nervous systems, predominately the digestive and respiratory tracts. While the current prevalence of NET in the US is ~150,000 patients (incidence of ~19,000 new cases per year), current treatment modalities are limited to subsets of NET with no broadly effective drugs across the NET spectrum. Data from Frost & Sullivan indicate the global NET market in 2018 was worth approximately $5.8bn and is expected to grow to $21.2bn by 2030. In particular, in China there is significant market opportunity with reported incidence of NET in 2018 of approximately 67,600.
Surufatinib is an oral angio-immunokinase inhibitor that targets VEGF1, 2 and 3; FGFR1 and colony stimulating factor 1 receptor (CSF-1R) kinases. CSF-1R is a cell-surface protein that acts as the receptor for the cytokine CSF1, which controls macrophage (a type of white blood cell) function. Inhibition of CSF-1R limits the production of pro-tumour macrophages, which, among other functions, is believed to aid in angiogenesis, tumour cell invasion and evasion of the immune system.
Following encouraging POC data from the Phase II study in biliary tract cancer (BTC), a pivotal open-label Phase IIb/III BTC trial (NCT03873532) in China has recently initiated, with the first patient dosed on 22 March; BTC represents a high unmet need due to limited treatment options and an increasing patient population. HCM is additionally developing the drug internationally: the global Phase Ib/II study (NCT02549937) in pancreatic NET (second-line in Sutent/Afinitor refractory cancer) and BTC started enrolling US patients in July 2018. These trials will be managed by HCM’s clinical and regulatory team that is now in place in New Jersey, US.
With the growth and widespread use of PD-(L)1 inhibitor in the treatment of solid tumours, clinical trials combining these immune checkpoint inhibitors (ICIs) with CSF1(R) inhibitors have been initiated by several multinational pharmaceutical companies. HCM has recently initiated a Phase I dose escalation study (NCT03879057) in combination with Shanghai Junshi Biosciences’ PD-1 inhibitor Tuoyi (toripalimab), which was recently approved in China for melanoma.
Peak sales potential of $1.0bn across all indications
We forecast global peak sales for surufatinib of $953m across the NET and BTC indications. We have removed thyroid cancer and adjusted sales forecasts. Our peak sales forecasts for surufatinib in NET ($169m China, $454m RoW) are conservative as at this point. We have updated our assumptions for NET in China and now assume an incidence of 68,000 (vs 50,000 prevalence rate previously) and 19 months of treatment (vs 12 months previously) in China. This is based on new data from Frost & Sullivan and Phase II median PFS data (19.4 months). However, we note a patient-access programme similar to Elunate could reduce the number of months HCM receive reimbursement for surufatinib. Additionally, following the positive SANET-ep Phase III data we have upgraded our probability of success in NET to 90% from 75% previously. We forecast peak sales of $187m in BTC in China and $143m in RoW.
Global development of HMPL-523 and HMPL-689
Longevity for R&D-driven biopharmaceutical companies is dependent on having a pipeline of innovative assets that span both indications and development phases. HCM’s strategy to date has focused on developing best-in-class kinase inhibitors that target solid cancers; the next wave of internally developed assets, HMPL-523 (Syk inhibitor) and HMPL-689 (PI3Kδ inhibitor), are kinase inhibitors, which have a clinical focus for various haematological (blood) cancers and autoimmune conditions. Concurrent Global and Chinese clinical programmes are ongoing, and recent IND approvals mean both assets have started US/EU Phase I clinical development for indolent NHL.
B-cell malignancies are a huge commercial opportunity
Haematological cancers arise in blood-forming tissue (leukaemias and myelomas) or in the cells of the immune system (lymphomas), such as B cells or T cells. Treatment options vary according to cancer type and genetic burden. HMPL-523 and HMPL-689 are being developed for a range of B-cell malignancies (Exhibits 12 and 13). The commercial opportunity in treating haematological malignancies is substantial. Many novel treatments have reached blockbuster status in haematological cancer sales alone, for example, Revlimid ($8.9bn), Rituxan ($5.4bn) and Imbruvica ($4.5bn) in 2018. The US National Cancer institute SEER data estimate that in 2018 there were c 170 000 new cases of blood cancers, 33% of which were leukaemias , 18% multiple myeloma (MM), 5% of Hodgkin’s lymphoma (HL) and 44% with non-Hodgkin (NHL), which comprises over 30 types of aggressive (fast) and indolent (slow) B-cell and T-cell malignancies. Five-year survival rates (2008–2014) range from 27.4% in AML to 71.4% in NHL as patients relapse or become refractory (r/r) to current treatment options. Efficacy and safety data from the ongoing Phase I studies of both HMPL-523 and HMPL-689 will further define in which patient populations these assets could have utility, as well as give an early indication as to whether they could be used in combination with other treatments (safety data).
The B-cell signalling pathway is critical in haematological cancers. Approved novel drugs that target this pathway include Imbruvica, Zydelig, Jafaki and Rituxan (Exhibit 11). Targeting aberrant B cells is not only a proven strategy for the treatment of B cell-related malignancies, but also in autoimmune disorders such as multiple sclerosis (MS) and rheumatoid arthritis (RA), highlighted by the commercial success of antibodies such as Rituxan (rituximab) and Ocrevus (ocrelizumab). HCM is evaluating HMPL-523 in idiopathic thrombocytopenic purpura (ITP), an immune-mediated disease that results in low platelet levels.
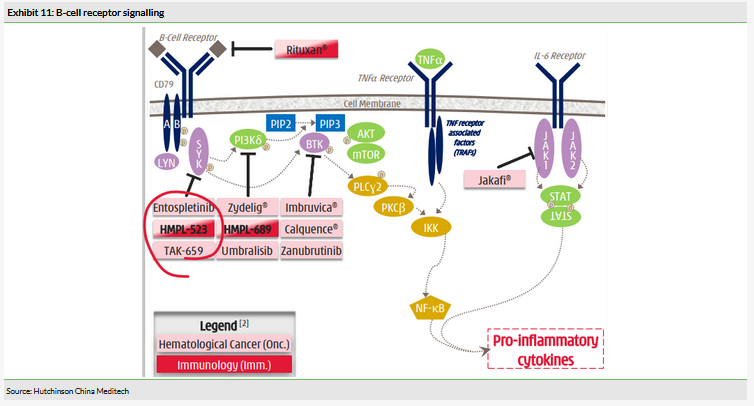
Both HMPL-523 and HMPL-689 are small molecules designed to target kinases in the same B-cell receptor signalling axis as the Bruton’s tyrosine kinase (BTK) inhibitor Imbruvica and Zydelig, which, like HMPL-689, acts through inhibition of PI3Kδ. Importantly, HCM retains the global development and commercialisation rights to both of these assets and both could present significant growth drivers in the medium and long term. We believe a key factor in success will be whether both assets can demonstrate good safety profiles as monotherapy as this will indicate potential combination regimes, which is where we believe treatment paradigms are shifting in treating B-cell malignancies. Likewise, a well-tolerated treatment will be essential for these to show any utility in autoimmune disorders. To date, HMPL-523 has been dosed in c 110 patients and c 118 healthy volunteers, HMPL-689 in c 30 patients and c 48 healthy volunteers and both have shown no off-target toxicity.
HMPL-523: First-in-class opportunity for blood cancers
HMPL-523 targets Syk, an enzyme believed to be involved in diverse range of biological functions including in autoimmune disorders and haematological malignancies. In particular, Syk is involved in BCR signalling and is thought to promote the survival and maintenance of malignant cells. Syk sits just upstream of BTK and PI3Kδ and mediates their activation, therefore targeting it presents an alternative way of treating patients who are resistant or intolerant to Imbruvica or Zydelig (Exhibit 11). The clinical landscape for Syk inhibitors remains relatively competitive and in 2018 the US FDA approved the first Syk inhibitor, Rigel Pharmaceutical’s fostamatinib (Tavalisse) for treating ITP. Entospletinib, developed by Gilead (NASDAQ:GILD), is one of the most advanced clinical candidates for treating haematological malignancies.
HCM believes HMPL-523 is a potential best-in-class molecule and potentially first-in-class for haematological malignancies and we anticipate that launch in China in 2023 is feasible. Preliminary data presented at ASH2018 from the ongoing the Phase I/Ib study in China showed some promising signs of efficacy:
- 21 evaluable patients, with relapsed or refractory disease (median of three prior treatment lines, predominately chemotherapy and CD20 targeting antibodies);
- 19% showing partial response (3/10 follicular lymphoma [FL], 1/3 CLL/SLL); and
- 40% achieving stable disease (3/4 mantle cell lymphoma [MCL], 3/10 follicular lymphoma [FL], 1/3 CLL/SLL, 1/2 marginal zone lymphoma [MZL], 1/1 Waldenstrom macroglobulinemia [WM]).
Click on the link to read the full report...