Orexigen (NASDAQ:OREX) faces a U.S. Food and Drug Administration (FDA) approval decision for NB32 (Contrave) on September 11th, 2014. Contrave is a combination of bupropion sustained-release (SR) and Orexigen´s wholly owned SR version of naltrexone, designed to help patients lose weight. The addition of Naltrexone said to improve patient tolerability for the treatment. The company is due to receive roughly $100 million with approval and launch of the product.
Over the last few years, weight loss drugs have received an abundance of media attention and investor speculation.
Arena's (NASDAQ:ARNA) Belviq gained FDA approval in June of 2012. Belviq is approved for use in adults with a body mass index of 30 or greater, or adults with a BMI of 27 or greater who have at least one weight-related condition such as high blood pressure, type II diabetes, or high cholesterol.
Belviq is thought to selectively activate serotonin 2C receptors in the brain, which may help a patients eat less and feel full after eating smaller amounts of food.
Belviq has its limitations on how it can be prescribed, and cannot be prescribed if a patient is taking other products intended for weight loss. This means that a patient cannot "mix" Belviq with other weight loss drugs. This limitation could potentially affect overall weight loss for a patient, and could dissuade both patients and doctors from prescribing Belviq.
Additionally, the effect of the drug on cardiovascular morbidity and mortality have not been established, which is a very important factor for both patients and their doctors who might be inclined to prescribe Belviq over other weight loss drugs.
Orexigen's Contrave seems to have one advantage over Belviq; its "Light Study" data. The Light Study interim data met the SPA requirements on 8900 patients and showed Contrave did not increase the risk of MACE. Doctors could be more inclined to prescribe Contrave over Belviq because of this cardiovascular study - there is no such study with Arena's Belviq.
Vivus's (NASDAQ:VVUS) Qsymia was approved by the FDA in July of 2012. However, Qsymia was approved with a Risk Evaluation and Mitigation Strategy (REMS), which informs doctors and female patients of reproductive potential about an increased risk of orofacial clefts in infants exposed to Qsymia during the first trimester of pregnancy. Even with this warning, Qsymia has been seeing decent script numbers of late. Starting in 2013, Qsymia scripts saw 81k in quarter two of 2013, 109k in Q3, 2013, 124k in Q4, 2013, and 121k in Q1, 2014, respectively.
However, of all the Qsymia scripts, 54% are discounted, of which 33% are sold at a discounted price along with 21% given away for free. We feel this is a bad way to do business with the safety issues that could arise from Qsymia for women. Both Arena (via Eisai) and Vivus are engaged in healthy promotion to get scripts up by giving away discounted and free scripts. There is no question that the demand is there from people who are clinically defined as being obese. The greater question is what is the longer term safety of both Belviq and Qsymia?
In clinical trials of Contrave in excess of 4,500 people, 53% of those taking Contrave and 21% of those taking placebo lost five percent or more of their body weight over the twelve month trial duration, with many patients seeing improvements in cholesterol levels and blood sugar control. For those who actually went on a proper diet and properly exercised, the results were predictably better.
If Contrave is approved, it will be marketed by Takeda Pharma (OTC:TKPHF), a large Japanese company worth in excess of $35B
Cardiovascular issues seems to be the number one concern with these weight loss drugs. Belviq and Qsymia seem to be unknowns in this regard while Contrave so far has the interim positive safety data from its Light Study.
The Light Study has successfully met the SPA requirements on 8900 patients showing that Contrave did not increase the risk of major adverse cardiovascular events (MACE). Because of this study, doctors might be more inclined to prescribe Contrave over Belviq and Qsymia, which both lack the cardiovascular (CV) data Contrave enjoys.
This factor seems to favor Contrave over Belviq and Qsymia, and could possibly tip the scales towards Contrave in the United States. Vivus and Arena failed to gain approval in Europe (Arena withdrew Belviq application) because both of these drugs simply did not have the data needed for European regulators to give it the green light.
Contrave has a very good chance to gain both FDA and European approval, with the expected European approval decision to occur sometime in October of this year. The political pressure could be ramping up in Europe to approve a drug like Contrave. In Europe, it’s estimated that new cases of diabetes will increase from 366M to over 550M by 2030.
In 2012, France, Germany, Italy, Spain, and the U.K. saw combined spending over 120B for diabetic treatments, and this number continues to rise. Obesity in Europe is now being considered a disability. With Belviq and Qsymia seemingly out of the game in Europe, if approved, Contrave could dominate that market region.
Orexigen will receive $100M upon approval and launch in the USA of Orexigen.
Bullish Call Option Chain:
August Options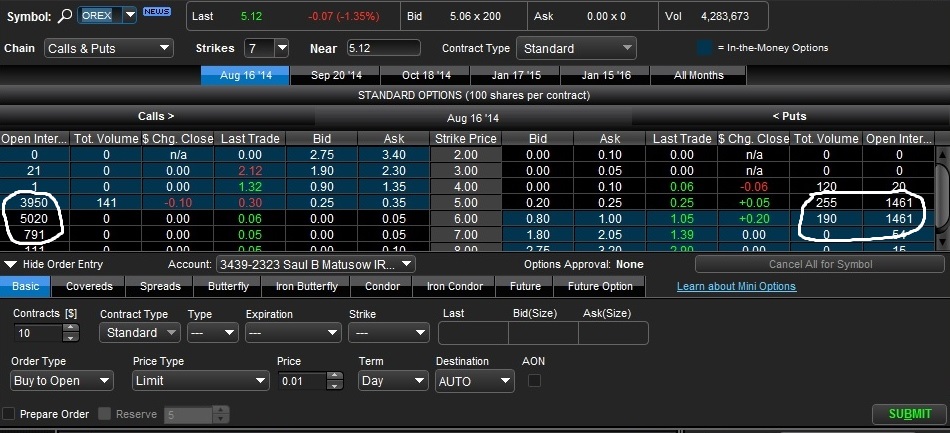
As we can see above, there is heavy open interest in the August calls, so some traders believe there will be an an early approval. The put side looks more like a hedge, and not a very big one.
September Options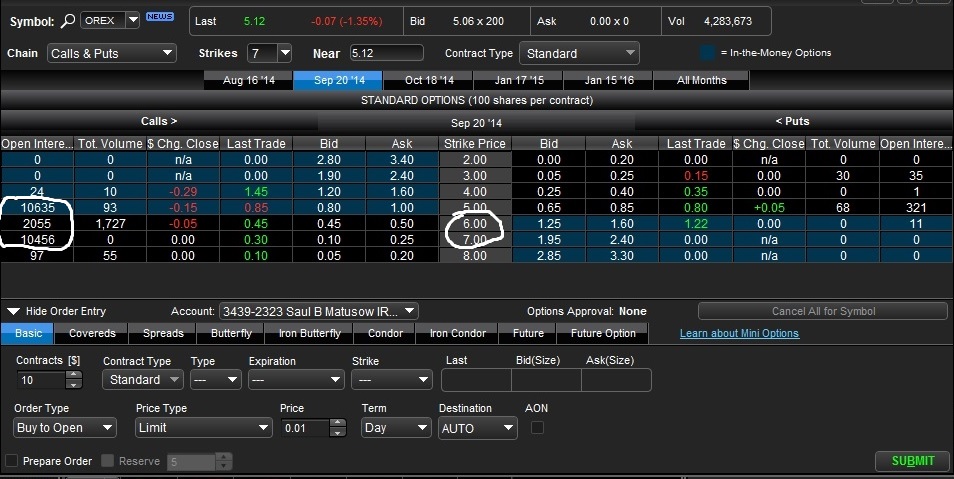
The September calls show an extremely bullish sentiment, which most investors and traders believe the stock will be near or over $7 by the time of the September expiry. Orexigen is taking the proper steps and engaging in proper communication with U.S. and European regulators to ensure approval for Contrave here. To show constrast, recently AcelRx received a complete response letter (CRL) from the FDA for its pain medication dispensing machine, Zalviso. AcelRx did not ask for an approval extension and "gambled" that the FDA would review the application amendments -- they lost that gamble and it has so far cost investors 30% equity loss. We can clearly see Orexigen is not willing to take that gamble which we think bodes well for investors.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Global Approval Could Be On The Horizon For Orexigen's Weight Loss Drug
Published 07/30/2014, 09:51 PM
Updated 07/09/2023, 06:31 AM
Global Approval Could Be On The Horizon For Orexigen's Weight Loss Drug
Latest comments
TRY ARENA;S CAMILLA STUDY. . WHY IS CONTRAVES 2 GENERICS AVAILABLE TODAY BEING QUESTIONED BY EU??. WHERE IS THE DATA FORM LIGHT STUDY. THE REAL DATA. .
Belviq has its limitations on how it can be prescribed, and cannot be prescribed if a patient is taking other products intended for weight loss. ?????????????????????????????. . so the belviq phenermine triasl is a fraud??. . BEL PHEN WILL BE THE BIGGEST BLOCKBUSTER SINCE FEN PHEN. . POOR SCOTTY. TAKING OVER FROM ADUMB?
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
